Waters Staff
Waters Staff is comprised of contributors with a variety of expertise across our organization.

Waters Staff is comprised of contributors with a variety of expertise across our organization.

In late January, I traveled to WCBP (Well Characterized Biological Products), an industry conference that is hosted every year by the CASSS organization. It serves as a forum that brings together biopharmaceutical industry professionals and various regulatory agencies from around the world to review and discuss the current and future state of biopharmaceutical drug development….
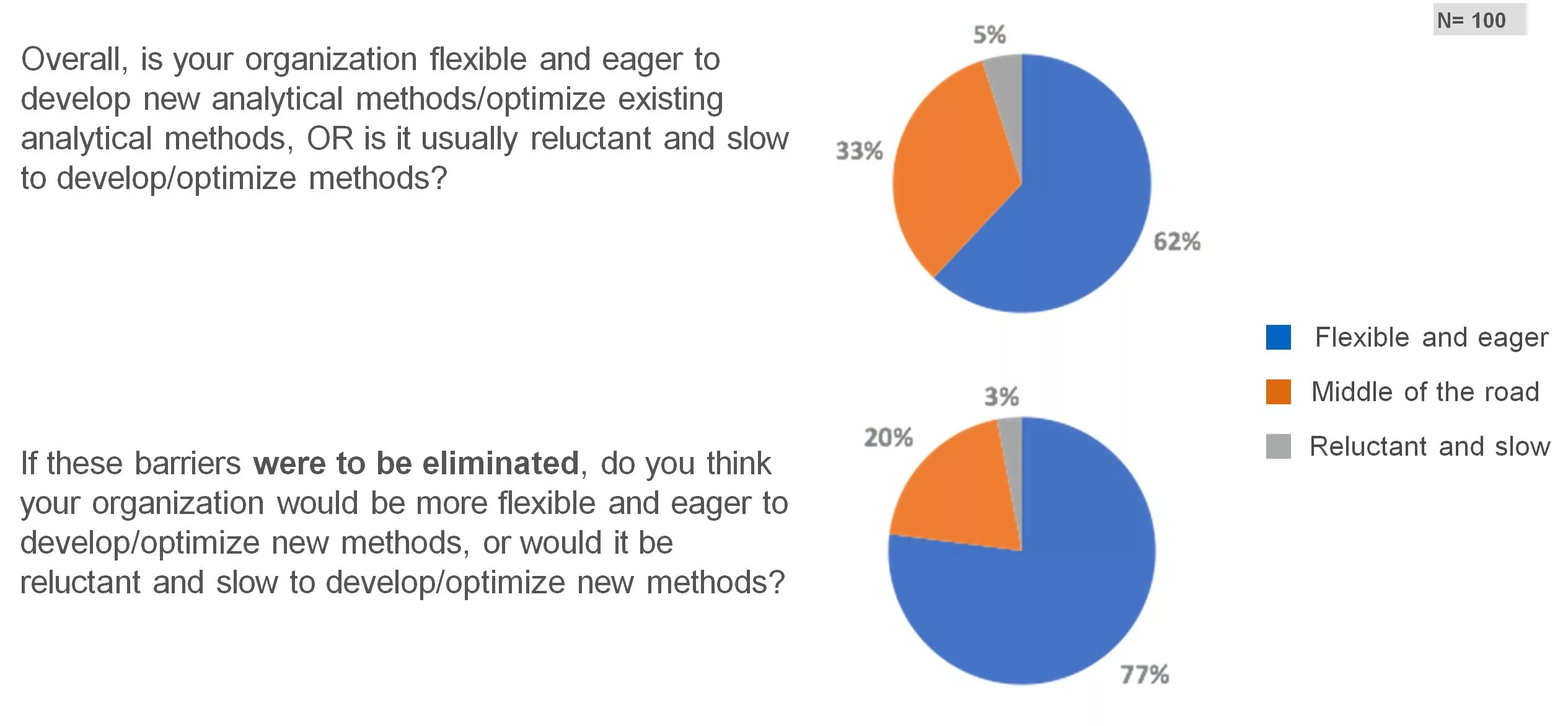
Waters recently surveyed 100 method developers from innovator and generic pharmaceutical companies to better understand their existing challenges and views on Method Lifecycle Management (MLCM). We’ll share with you the top questions and findings from our survey results.

Waters’ legacy of purposeful innovation continues with the recently introduced RenataDX Screening System, a flow-injection tandem mass spectrometry system for high-throughput analysis of extracted dried blood spots and other physiological matrices.

Opening this week in Singapore is a new resource for scientists in the challenging position of needing analytical insights as part of their food and water research, but who lack access to state-of-the-art instrumentation … we’re so excited we wanted to share a preview!

There are two primary multi-attribute monitoring (MAM) assay choices for biologic development and QC: subunit protein mass analysis and peptide mapping by LC-MS. Here we explore how peptide mapping LC-MS MAM workflows are being used.

Q&A from a recent forensic toxicology webinar on using an ACQUITY UPLC I-Class/Xevo TQ-S micro MS system for quantifying cannabinoids in oral fluid.

Two MAM approaches for biotherapeutic analysis are being implemented today; one focused on the analysis of monoclonal antibody (mAb) subunits, and the other focused on the analysis of peptides from a protein digest (peptide mapping workflow). Both have their advantages and disadvantages. Here, we explore MAM for mAb subunit analysis.

The improvements in LC-MS technology under GxP compliant-ready informatics for biopharmaceutical analysis has prepared the industry to expand the role of MS technologies beyond product characterization toward monitoring product and process quality attributes.

Where does your service engineer turn if they need help? Meet Dipesh. He works in Waters’ Global Service Support (GSS) department in Wilmslow, UK. The team supports field service engineers, writes supporting documentation, and advises on service requirements for new products.

Meet Vicky, who joined Waters’ Customer Success Team about two years ago with 20 years of LC-MS experience. She specializes in clinical forensic and toxicological applications supporting customers in Northern Europe.

You won’t like what we found when we tested skin lightening cosmetics that we purchased online.

Meet Ian, an LC-MS system engineer based in the UK, who is responsible for system installs, service, and maintenance. Being a former Waters customer drives Ian’s approach to customer service and going the extra mile.