Chapter and Verse: USP 621 and You

In August 2014, the United States Pharmacopeia and the National Formulary (USP-NF) put into effect new guidelines to “allowable adjustments” in its Chapter <621> (USP37-NF32 S1). And as of August 1, 2016 when USP 39 NF 34 S1 goes into effect, for <621> nothing significant has changed since.
The USP doesn’t publicize changes to their guidelines in an overt way, but these changes were announced on their website well before the new guidelines went into effect.
They are not regulations, per se. But, in the US at least, the US Food and Drug Administration (FDA) treats them as parameters for their regulations.
So, like a pedestrian walking the tracks in the path of an oncoming train, it’s probably best to heed the flashing lights, lest you find yourself on the wrong end of 10,000 tons of quickly-moving steel.
(Spoiler alert: The train always wins.)
USP Chapter <621>, you say?
The USP-NF is a book of pharmacopeial standards that has been designated by the FDA as the official compendia for drugs marketed in the United States. Its chapter on Chromatography is <621>, found here, and “defines the terms and procedures used in chromatography and provides general information.” Specifically, it explains the allowable adjustments to chromatography systems in order to meet system suitability requirements.
A guide to guidelines.
The recent changes have been influenced by a 2009 stimuli paper written by Dr. Uwe Neue et al, which proposed scientifically-based applications of scaling methods. While this paper suggested changes to method transfer of both isocratic and gradient methods, the USP has implemented the changes to isocratic methods, only.
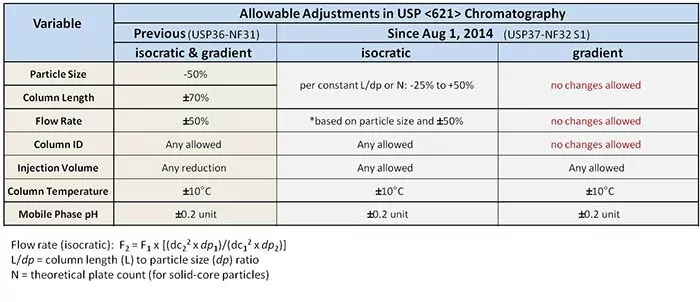
Previously, changes pertaining to particle size were allowed, presuming the changes were reduced within 50%. Column lengths could be increased or decreased 70%, flow rates could be increased or decreased 50%, and the column I.D. could change at-will. Flexible? Yes. Well, seemingly – but, a 50% reduction in a 5-µm particle size only brings us to 2.5 µm, not the newest sub-2-µm particles.
Now, for isocratic methods, changes in particle size and column length are treated as a ratio, rather than discreet parameters, that can decrease 25% or increase 50%, while flow rate can still increase or decrease by 50%. Any changes made to column I.D. are allowed. Depending on your column length, the newest sub-2-µm particles may now be available for use, validation-free.
However, for gradient methods, no changes are allowed in any of the following: particle size, column length, flow rate, or column I.D.
Still with me?
See the handy table below. Because, pictures.
Thanks for the picture, now what’s this all mean?
By staying within the parameters of “allowed adjustments” of isocratic methods, analysts can simply perform verification of method performance and avoid time-consuming revalidation steps.
And so…
And so chromatographers now have greater ability to implement the newest column technology while still adhering to existing monographs. This means, in short, the opportunity to adopt more forward-thinking methods in the laboratory that, in the end, can lead to reduced solvent consumption, faster analysis times, and an overall increase in ROI.
No, you won’t be met with impending doom if you don’t adopt UPLC/UHPLC.
At least, not yet. For those running isocratic methods, the path to a UPLC platform is a brightly-lit one. Per a white paper released by Waters, “the flexibility in the new guidelines now opens the possibility to transfer previous isocratic HPLC methods to UPLC without the time investment and hassle of revalidation, while realizing the overall laboratory efficiency and productivity gains of higher throughput assays.”
Put more simply, harnessing sub-2-µm particle column technology can result in almost 10-fold time savings and greater than 15-fold decrease in solvent consumption. Add to that tools such as the Empower 3 Method Validation Manager (MVM), which allows you to perform chromatographic method validation – from protocol planning through final reporting – in one application, and cut method validation time and cost by as much as 80%.
I’ll let you do the math. Or save yourself the time, let Waters do the math for you.
Alas, gradient methods are more popular in their usage.
However, given the new guidelines, any changes to column configurations for existing methods now require full revalidation.
When a method fails to meet system suitability requirements? Revalidation.
Currently using a compendial method with modifications that were previously allowed? They’re not anymore.
At least, not without revalidating.
Now, this is not a reason to go all Office Space on your HPLC system and then run out to buy a UPLC platform. (If for whatever reason you do choose to go this route, make sure you get it on video. You know, “viral.”)
But, while HPLC still has excellent robustness, and you can still continue running legacy methods without having to make any major shifts in your technology, it might be worth considering revalidating on the best tools available. Doing so on old technology could lead to wasted time and resources.
Fun with acronyms – CQA and QbD.
By conceiving UPLC a decade ago, Waters allowed customers to not only go faster, but also to see more.
Seeing more means being able to better recognize Critical Quality Attributes (CQAs), so that analysts can better understand products. Having access to better information means having the ability to make better decisions.
This all ties into a Quality by Design (QbD) approach to a product, in which quality is built into both the product and the process, mitigating risk in the manufacturing and analysis of the product.
And now after the lesson in alphabet soup, what’s this got to do with you?
Frankly, the above-referenced steel train. Also, not to mention, your customers.
The FDA has been putting out guidance that analysts should start thinking more about the life cycle of their methods. For example, if a method continues to not meet system suitability requirements, perhaps it’s time to move to newer technology, such as UHPLC or UPLC, in order to gain a better understanding of that method.
In short, understand what’s in the sauce, and stop just following the cookbook.
Consumers also ultimately benefit from laboratories moving to new, high-resolution technology and being able to know more about a product. Several cases involving the use of UPLC technology in discovering previously unknown dangers in products have become highly publicized over the past decade, including melamine in infant formula and complications/deaths related to heparin.
Science doesn’t stop moving forward.
And with it, neither do the regulations. This step by the USP toward modernization of methods introduces an opportune point of consideration with respect to the use of time and valuable resources for laboratories and, ultimately, businesses concerned with their technology lifecycles and related return on technological investments.
Additional resources:
- Future-Proof Solutions for Regulated Laboratories In the Face of Changing USP <621> Guidelines
- Dwell Volume and Extra-Column Volume: What Are They and How Do They Impact Method Transfer
- USP Method Modernization Using “Equivalent L/dp” and “Equivalent N” Allowed Changes with Solid-Core CORTECS C8 Columns
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (22) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (20) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)