Have we bridged the gap between qual and quan using HRMS?

Increa sing Size, Complexity and Potency: The Bioanalytical Challenge of the Next Decade, was the theme for the 3-day APA India held in Mumbai, India from February 23-25, 2015. Close to 300 attendees gathered to discuss current trends and to offer strategies for overcoming these challenges moving forward.
sing Size, Complexity and Potency: The Bioanalytical Challenge of the Next Decade, was the theme for the 3-day APA India held in Mumbai, India from February 23-25, 2015. Close to 300 attendees gathered to discuss current trends and to offer strategies for overcoming these challenges moving forward.
The second session of the morning was Bioanalysis: Large and Small Molecules. In his talk titled, ‘Applications of Novel Acquisition Modes and Instrument Geometries in Time-of-Flight Mass Spectrometers for Targeted Quantitation,’ Stephen McDonald, Pharmaceuticals Strategic Marketing Manager in Waters’ Worldwide Marketing Group, presented on the current status of High Resolution Mass Spectrometry (HRMS), setting the stage for a discussion around the utility of HRMS for quantitation.
For all its advantages – specificity, accurate vs. nominal mass, full scan sensitivity – HRMS is traditionally employed in qualitative studies and less so for quantification. This is primarily because of the technology’s historical inability to reach the desired limits of detection as compared with tandem quadrupole mass spectrometry. Enter Waters’ newest Time of Flight (TOF) instrument; the Xevo G2-XS, launched at ASMS in 2014. With a redesigned, segmented quadrupole collision cell, ion transmission efficiency is significantly increased so that nearly all ions enter the TOF, which has a dramatic impact on sensitivity. Data presented highlighted the comparison of the new G2-XS with its predecessor, the G2-S, with 4-fold improvements in LLOQ, reaching down to low double-digit pg/mL levels for small molecules. With the use of Tof MRM and Target Enhancement – an MS method that borrows from the lessons learned with tandem quadrupole instruments and mimics their geometry – LLOQs of single digit pg/mL were attained. The same experiments performed on the G2-XS coupled with ionKey/MS – an integrated microflow device – yielded subpicogram levels of detection, a nearly 8-fold improvement vs. G2-XS alone, and an almost 20 fold improvement relative to the original G2-S method.
The implicatio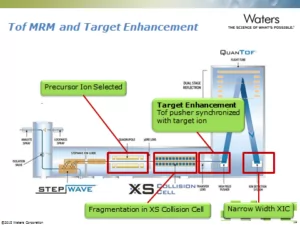 ns? With HRMS already being used in the qualitative arena, such as in the characterization of Antibody Drug Conjugates (ADCs), there is the potential that HRMS could bridge the gap between the current qualitative LC/MS methods and quantitative Ligand Binding Assays. Bringing these disparate methods together into a single analysis could dramatically improve efficiency when studying ADCs.
ns? With HRMS already being used in the qualitative arena, such as in the characterization of Antibody Drug Conjugates (ADCs), there is the potential that HRMS could bridge the gap between the current qualitative LC/MS methods and quantitative Ligand Binding Assays. Bringing these disparate methods together into a single analysis could dramatically improve efficiency when studying ADCs.
During the panel discussion that followed this session, one question from the audience was the existing resistance to this technology in a regulated environment. There was agreement from the group that these were the same issues encountered when bioanalysis transitioned from UV-based assays to tandem quadrupole mass spectrometry. In order to address this concern, it falls to the broader community to set the standards surrounding HRMS, and concomitantly, it is the responsibility of the instrument manufacturers to make these instruments increasingly accessible and easy to use.
With the industry shift from small to large molecule bioanalysis, especially for ADCs where there are multiple parameters that need to be addressed for a single entity, HRMS may very well be one of the tools that the bioanalytical community turns to in order to address these challenges.
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (22) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (20) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)