Maximizing LC Column Lifetime: 3 Tips to Reduce Chemical Deterioration

Leave it in the box? Store it in mobile phase? Read the manual?
All chromatographic columns have a finite lifetime that is highly dependent on its care and use. Even under the best of conditions, HPLC and UPLC columns deteriorate over time due to chemical or particulate contamination.
Many of the limitations in column lifetime are not due to the column, but are caused by other factors that can be attributed to either chemical deterioration or physical contamination. Some of these factors are unavoidable and are part of normal usage.
Achieving the maximum lifetime and performance from your analytical LC column can be accomplished by following 3 simple recommendations to reduce chemical deterioration.
1. Know your column’s operational conditions
I know this sounds obvious, but following the manufacturer’s guidelines for operational ranges for column temperature, mobile phase pH, and buffer additives will greatly improve column lifetime and performance.
Column manufacturers extensively test columns over a wide range of operational conditions to ensure robustness and good performance. Fortunately, this information is included in the care & use manuals of most columns. Pay special attention to the “Column Use” section of the column manual, which typically covers sample prep recommendations, ph Range guidelines, information on solvents, as well as maximum operating pressure advice.
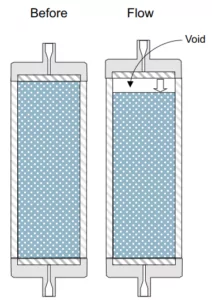
For example, silica-based reversed-phase packings are very stable at room temperature for most mobile phase conditions between pH 2 and 8; hybrid-silica particles extend the operational pH range from 1-12. However, if you increase the temperature, especially when you are working close to the pH limits of the packing material, column life may deteriorate rapidly. This poses problems, especially for many USP methods that operate close or near pH 7. Combine these conditions with elevated column temperatures and you will most likely have an early column failure due to dissolution of the silica-based particle.
This commonly creates a void in the stationary phase at the column inlet resulting in distorted peak shape and in extreme cases split peaks.
At lower mobile phase pH conditions, the ligand can potentially hydrolyze and cleave from the stationary phase surface resulting in increased peak tailing and reduced analyte retention.
The choice of the buffer ions used in the mobile phase plays a significant role in column degradation. Phosphate buffers are commonly used but are not the best choice; significantly better results can be obtained with organic based buffers like TRIS or citrate. In general, organic-based buffers are less aggressive towards the silica-based surfaced. The buffer concentration contributes to the column lifetime: lower buffer concentrations result in improved column life.
In some cases, operating at the extremes is unavoidable. The separation conditions need to be balanced against the ruggedness of the assay; however, even under more stressful conditions, good column lifetimes can be achieved when following manufacture guidelines.
2. Ensure proper column equilibration and conditioning
Column equilibration is straightforward with the equilibration time being dependent on mobile phase composition and concentration. In general, more concentrated mobile phase conditions require less equilibration time compared to more diluted compositions. This is due to the kinetics between the mobile phase and stationary phase with more dilute mobile phases imparting a lower surface concentration on the stationary phase.
The key factor for column equilibration is mobile phase volume, not time. Typically, it is recommended to use 5 to 10 empty column volumes for proper column equilibration.
There are differences between column equilibration and column conditioning — these two terms shouldn’t be confused.
Column equilibration comprises all phenomena that are reversible, while column conditioning changes the column irreversibly. Column conditioning can significantly impact column lifetime. When you condition a column you are changing the product that the manufacturer has delivered to you, and the reproducibility of this step is your responsibility.
It is generally advised against conditioning, but there are some circumstances where it’s unavoidable. For example, many ion-pairing separations require significant column equilibration and conditioning before stable retention times result. In this case, the additives interact with the stationary phase surface to impart the desired chemical affect. For this reason, columns that have been conditioned in this manner should be dedicated to that specific assay and separation conditions.
Once conditioned, the column will rarely work reliably for general purpose separations and should be avoided for developing new methods.
3. Proper storage of the column
There are many different ways to store an HPLC column that have little effect on column longevity. Choices in storage conditions are quite broad and can be based on convenience.
The most convenient way to store the column is in the mobile phase in which it is commonly used. The biggest advantage of this approach is that equilibration of the column with the mobile phase is very fast. This approach is especially recommended for normal-phase chromatography, where changes to the storage solvent can result in lengthy equilibration.
You do need to think this approach through carefully. The ideal conditions to store a chromatography column depend on the type of the stationary phase. Bonded-phase columns often change slowly when stored using common mobile phases and additives. Therefore, the convenience of storing the column in the mobile phase needs to be balanced against the reduction in column life. Commonly used C18 and C8 columns are sufficiently stable to be used for several months with little change in their hydrophobicity. However, bonded phases based on shorter chains can hydrolyze measurably within a few weeks.
The best storage conditions for reversed-phase columns depend on the frequency of their use. If the column is used daily, or even every few days, it is most convenient to leave it in the mobile phase used for the separation. For longer term storage, many column manufactures recommend to flush the column of buffer salts.
Related to storage is column “washing” or using a flushing solvent that is supposed to dissolve the adsorbed contaminants – beyond removing the mobile phase buffers – to extend column lifetime. In many cases, this more aggressive process simply does not work. For example, if the contaminants are proteins that have precipitated on the column, by the time you try to wash them off they have aged by denaturation or by cross-linking that makes them impossible to solubilize.
More related to column storage, every washing or solvent change also removes hydrolyzed bonded phase, which otherwise remains in a local equilibrium at the site where the hydrolysis occurred. Frequent and unnecessary flushing can actually result in an accelerated aging of the column by stripping away bonded ligand.
The tips presented above address common chemical interactions that are often overlooked but remain important to maximize column lifetime.
More resources:
- Maximizing LC Column Lifetime: 3 Tips to Reduce Physical Deterioration
- Looking for your Waters HPLC or UPLC column’s Care and Use Manual? Start here.
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (23) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (21) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)