Traceability in OneLab: OneLab configuration by the Lab Administrator

Bringing increased confidence to sample preparation workflows using automation, smart laboratory tools and electronic documentation for recording analyst activities
This article was revised in September 2023 to include important updates.
Consistency, accuracy, compliance, and data integrity can each be improved with automation of laboratory processes. We know that automated systems can not only reduce human errors when performing calculations but can also make documentation and traceability of result creation a simple process requiring only setting up and validation of the automated calculations. Traceability and accountability for sample preparation is achieved today with manual documentation activities in paper lab books and instrument logbooks. Not only are these tasks tedious for the analyst and prone to errors but tracing the “story” of the steps taken by the analyst for review purposes and to ensure accurate lab work is a big challenge for a lab supervisor.
When electronic documentation is leveraged, these tasks can be simplified for both the analyst and the reviewer while providing higher confidence in proper SOP execution. Technical controls such as time stamps, attributable analysts’ identification, equipment calibration checks, and detailed use logs can all be made available to document and track actual laboratory practices.
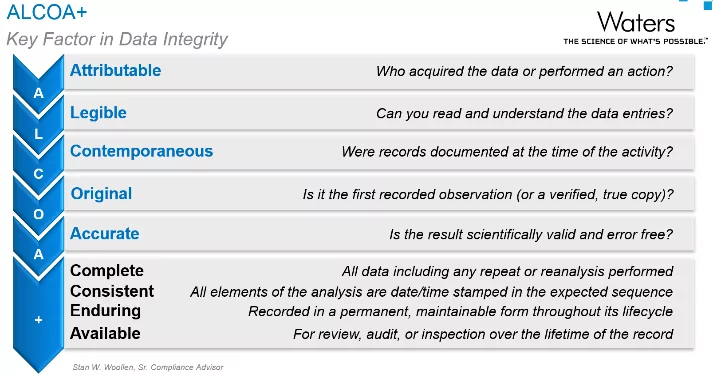
These technical controls are critically important at the ‘entire system’ level that is governed by the designated Laboratory Administrator in OneLab. It may be part of their job to document, and demonstrate to internal and external auditors, that technical controls required by electronic record and electronic signature (ERES) regulations (such as 21 CFR Part 11, EU Annex 11 and ISO 17025) are implemented. It is also important that procedural and administrative controls are in place to ensure user actions are controlled and recorded, and that data are secure, to meet the ALCOA+ definitions of data integrity.
Deploying any software tool designed to trace the actions of laboratory analysts performing experiments first needs some level of configuration: who are the analysts, what actions are they allowed to do, and which instruments or devices will they need to use. Traditionally, just capturing and keeping up to date information about instrument inventories can be a time-consuming activity. In addition, GxP regulations for Data Integrity concepts like ‘attributability’ need to be considered.
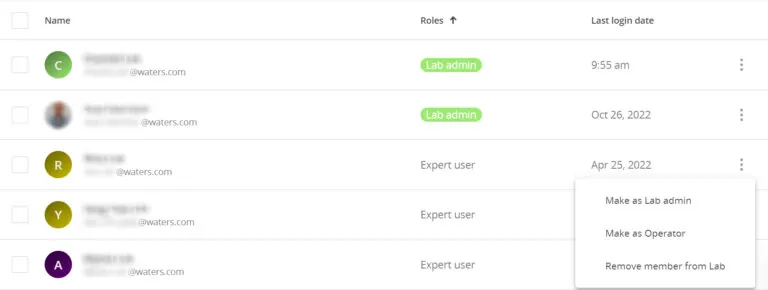
User management
The OneLab user roles are very intuitive. While the Administrator role can create users and data folders, and the Expert role can create or modify experiment protocols, the Operator can only follow a predefined protocol, which provides guidance in the correct use of the software and the laboratory instruments. Users of OneLab, even in the Administrator role, can never delete data or records, which makes meeting regulatory requirements of “completeness” easy.
Teams of OneLab users can be assigned to specific “Lab Workspaces,” allowing them to share devices, tools, protocols, and experimental details. This also manages the visibility to other users’ experimental work.
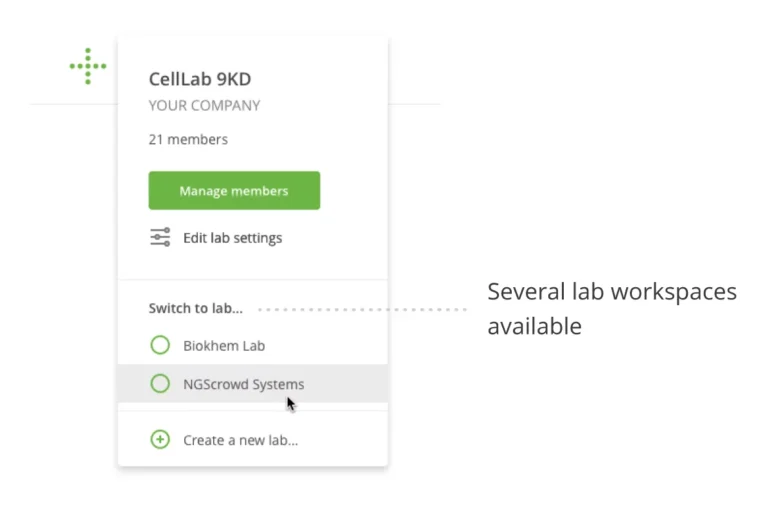
Audit trail for Lab Administrator
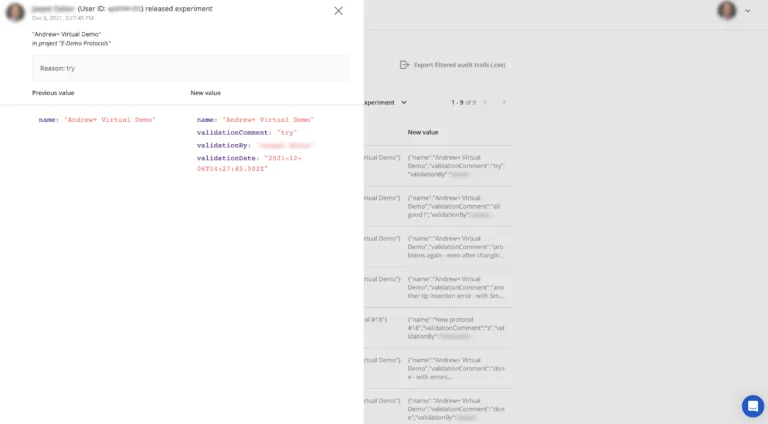
Just like all user activity in a regulated laboratory, it is also essential to record the activities of a Lab Administrator. All user activity in a specific Lab Workspace is viewable in the application. Expert and Operator members will see a high-level log of activities that are relevant and useful for their work. Users with the Lab Administrator role have access to a detailed audit trail of every activity that happens in the Lab Workspace.
If required, these audit trails can be exported for reading offline.

Archiving
A specific task assigned to the Lab Administrator role is to manage “archived records” for the Lab Workspace. Over time, any Lab Workspace may contain thousands of Experiment Records and hundreds of Protocols. Archiving these to a secured hidden location can simplify the use of OneLab – by only having current Protocols or recent Experiment Reports viewable while also ensuring that these archived records are close at hand if anyone needs to view them.
Archiving records and restoring them back to the Laboratory Workspace are both fully audit trailed processes. Data is not removed from the system and is still part of any active backup routines.
Device management
Maintaining accurate instrument logbooks is a big challenge for most laboratory staff. Even with electronic solutions, entering information about serial numbers, firmware versions and calibration cycles can be tiresome at system set-up, and keeping it current and accurate is an easily overlooked chore. With smart connected tools and devices, much of this critical meta data can be captured automatically in OneLab, with new connection information also automatically recorded in the device audit trail. For example, users can be instructed to check the last calibration date of a pipette before using it, saving time in having to repeat work that deviates from the protocol.
Additional information such as capturing vendor maintenance, calibration tasks or daily “suitability checks” can also be recorded in OneLab via execution of suitable protocols. Altogether, this documentation is used to ensure that the instrument or tool is performing properly before it is used for any lab work.
Once the instrument inventory is set up, the lab experts and analysts can go to work, with confidence that any records are created “contemporaneously”, and that specific actions and records are “legible” for the reviewer. Because OneLab software traces the use of each instrument or tool, instrument logbook histories are created automatically in the device management interface, without the need to manually document their use. Electronic logs capture details of each experiment the instrument was used for, by which laboratory analyst and records any issues that may arise. In addition, a detailed technical log for each connected device is always available and can be exported to assist in troubleshooting.

Platform management
When laboratories are using the SaaS cloud deployment of OneLab, platform level administration is provided as a service by the OneLab team. Tasks such as managing device licenses, revoking user access, back up, and upgrades are executed by the OneLab team, relieving your own administration teams from these responsibilities.
However, some customers choose to deploy their own instance of OneLab (either on physical servers, but most often in private cloud deployments) to have more control over the software. This solution is called OneLab Enterprise and, in this case, these platform administration tasks become the responsibility of the laboratory’s company. The actions of even a Platform Administrator are captured in a viewable audit trail, a capability introduced in 2020.
Conclusion
The compliant-ready OneLab software, along with smart connected devices and tools, makes configuration and set-up of laboratory methods and their traceability simple and foolproof. The user configuration is straightforward and allows the software to trace each analysts’ actions as the software is used. The instruments generate their own logbooks automatically based on the analysts’ use, without the need to manually write everything in an error-prone paper or electronic record.
Related Content:
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (22) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (20) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)