Traceability in OneLab: Protocols Designed by the Expert Analyst

Bringing increased confidence to sample preparation workflows using automation, smart laboratory tools and electronic documentation for recording analyst activities.
This article was revised in September, 2023 to include important updates.
Typically, expert staff in a lab will be responsible, along with Quality Assurance, to design and document a standard operating procedure (SOP) for other analysts to follow for routine sample preparation tasks. These procedures are key for repeatability and accuracy of laboratory experiments. Therefore, SOPs are important laboratory documents, which need to be issued and revised in a controlled manner with review and approval before use. All staff are trained on SOPs, as these form the pillars of laboratory quality. Analysts are then expected to follow the steps exactly, and document, traditionally in lab notebooks or paper worksheets, that the process was adhered to.
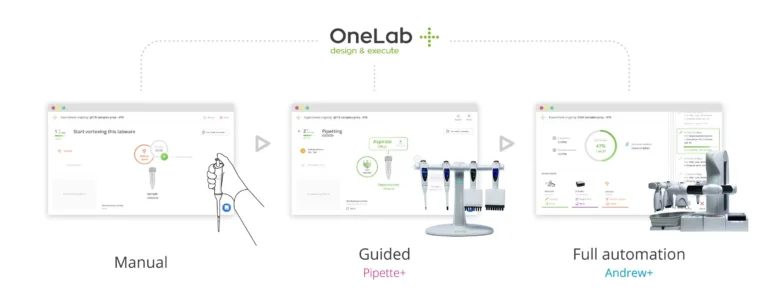
With the compliant-ready OneLab cloud-native software, user privileges, folders and devices are configured by the administrator. A user with the “Expert User” role can then securely log in and design a new protocol for analysts to follow. This protocol will be available to all analysts in a designated OneLab Lab Workspace, regardless of their OneLab user role. A single protocol has the flexibility of being executed entirely manually, via guided pipetting with Pipette+, or automatically by the Andrew+ pipetting robot with very little assistance from the analyst.
Traceability goals for protocol design
For the highest levels of traceability, the more detailed the protocol, the better. For automated execution using the Andrew+ robot, detail is critical, and appropriate validation testing would be required before releasing this protocol for general use.
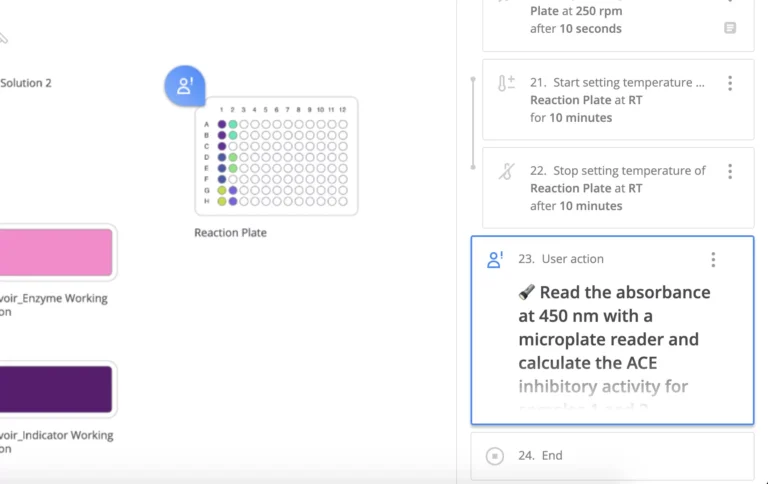
However, when executing the protocol with guided pipetting using the Pipette+ toolset, precise details are also useful to ensure that analysts can execute protocols with few errors. Protocols can not only define the steps that the analysts need to follow, but can also include informative text, videos, and pictures to help an analyst set up an experiment and show them exactly which devices or tools should be used, including model number and even serial number of the device, if required. A protocol can also include instructions to verify calibration dates to prevent the use of non-calibrated equipment.
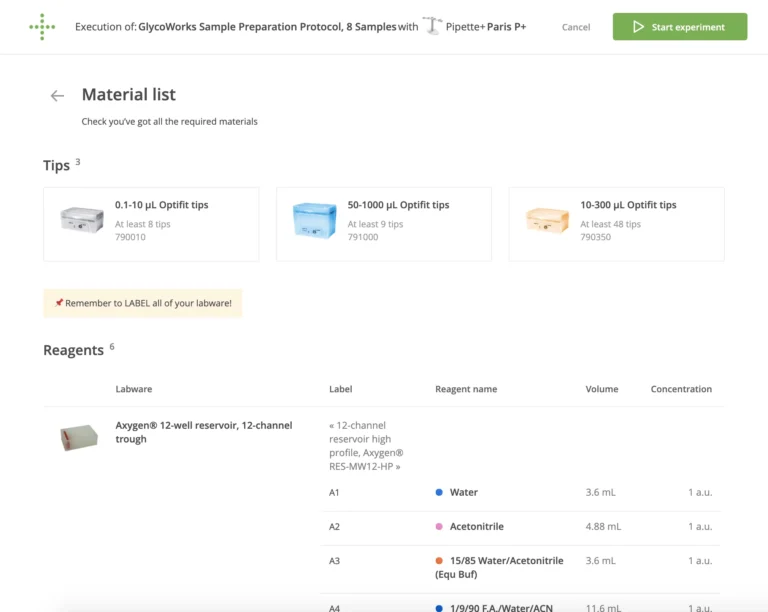
An expert user might also preconfigure a suite of reagents, including critical meta data about the reagent (composition, who it was prepared by, preparation date, expiration date, etc.) as well as the labware and devices to be used.
By specifying the details of these reagents in a protocol, there will be a higher degree of confidence that analysts do not make mistakes in either documenting the details or using the wrong reagents.
In regulated laboratories, contemporaneous documentation is key for data integrity and compliance
A protocol will normally be designed to prompt the analyst to document certain actions or critical values. In regulated laboratories, contemporaneous documentation is key for data integrity and compliance. However, in any laboratory, it is much easier and less error-prone to allow users to take notes as they perform activities rather than rely on their memory when they get back to their desks.
Traceability in -protocol design
Protocol design may be a collaborative task, with multiple expert analysts contributing to its completion. All changes to a protocol are recorded in the audit trails and associated with the corresponding user. In addition, users can also input comments or reasons explaining the changes they make. This is essential to satisfy GLP regulations and, at the same time, provides simple but useful documentation benefits.
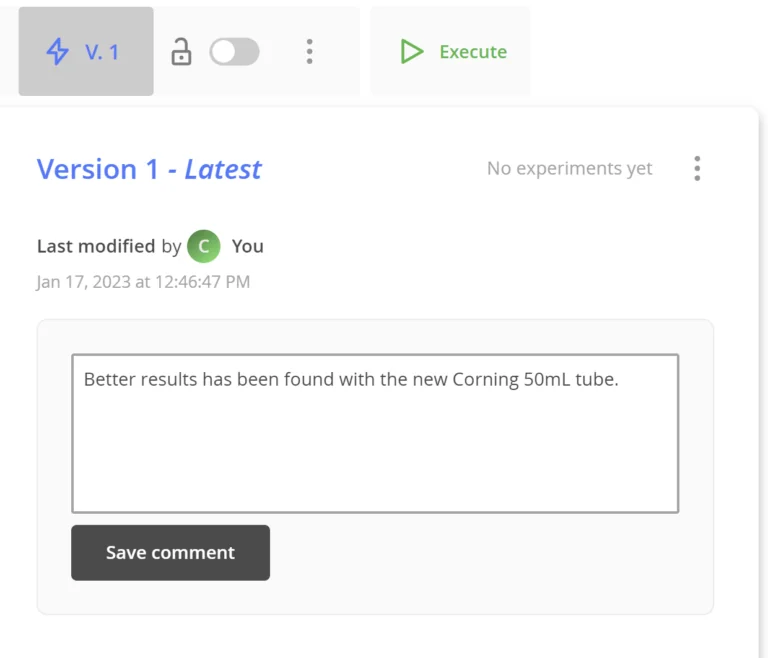
The entire history of the development of a protocol is available in the transparent version history built into all OneLab protocols automatically.
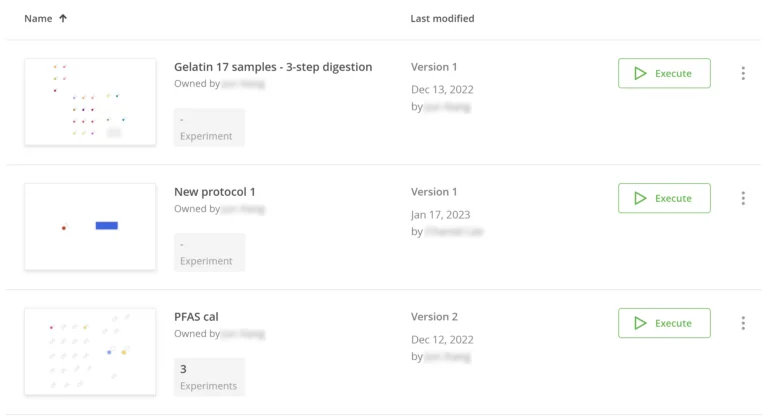
If, for any reason, the Expert users deem that a protocol needs to revert to an earlier version (if a new protocol step under test was deemed unsuitable for instance), they can do so in a few clicks while recording their reasons. Naturally, this action is fully traceable in the audit trail as well and automatically generates a new version of the protocol.
Verifying and locking protocols
As with highly automated protocols for the Andrew+ robot, protocols intended for analysts using guided or manual pipetting should also be verified before being released for use. This will typically involve some level of documented testing to be sure that the protocol includes all the steps required for the experiment before it is approved for use in the laboratory.
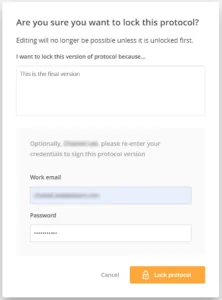
Once a protocol is fully designed and validated, the protocol is unchangeable – as would be expected – regardless of the user role. As detailed in typical GxP regulations, the publication or issuance of a protocol or other document containing instructions often requires the use of a signature to indicate official release.
To this end, Onelab allows Expert users to lock a protocol in its ‘final’ validated or verified state. This action of locking a protocol is recorded in the audit trail and may be accompanied, if required, with an electronic signature.
Should the protocol need to be unlocked to allow for future changes, that action is recorded in the audit trail, and a new version will be created.

Conclusion
Programming the Andrew+ robot or guiding an analyst through a validated protocol with assisted automation, will not only increase throughput and repeatability in a laboratory, but also create very detailed and automated documentation of steps taken by analysts. This documentation simplifies the role of the lab supervisor or reviewer who relies on this traceability to review the completeness and quality of experiments.
In addition, many diverse protocols are available in the OneLab Protocol Library. These range from highly detailed validated protocols for specific sample preparation tasks, to protocols designed as a starting place to inspire Expert users to develop protocols specific to their needs or simply common tasks such as dilutions.
Are you wondering how easy this is to set up? Does it take a long time to configure? See Traceability in OneLab: OneLab configuration by the Lab Administrator, which describes both user and device configuration.
Related Resources:
- Traceability in OneLab: Tracking the Analyst’s Completion of an Experiment
- For examples of OneLab protocols, browse the OneLab Protocol Library
- Try out OneLab for free
- Videos about OneLab
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (22) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (20) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)