Traceability in OneLab: Tracking the Analyst’s Completion of an Experiment

Bringing increased confidence to sample preparation workflows using automation, smart laboratory tools and electronic documentation for recording analyst activities.
This article was revised in September, 2023 to include important updates.
Laboratory employees appreciate the importance of traceability in their analytical work, whether to simply check for quality, or for regulatory reasons. Electronic documentation of laboratory activities can ensure consistency, accuracy, legibility, and availability of experimental records. However, manual entry in an electronic document is as onerous as writing in a paper notebook. By automating the documentation as much as possible and removing the burden from manual traceability processes, records are more accurate, more complete, and less prone to errors.
Tracing analysts’ work to comply with GxP Regulations
OneLab software provides traceability for the analysts’ activities, making it simple for reviewers to have confidence that experiments were executed consistently as outlined in the standard operating procedure (SOP). OneLab is compliant-ready because it includes the technical controls mandated by pharmaceutical and non-pharmaceutical laboratory requirements, such as electronic record and electronic signature (ERES) regulations (e.g. ISO 17025, EU Annex 11 or 21 CFR Part 11). When these technical controls are combined with procedural and administrative controls, including computer validation/assurance evaluations, OneLab can be used in GLP, GCP and GMP laboratories.
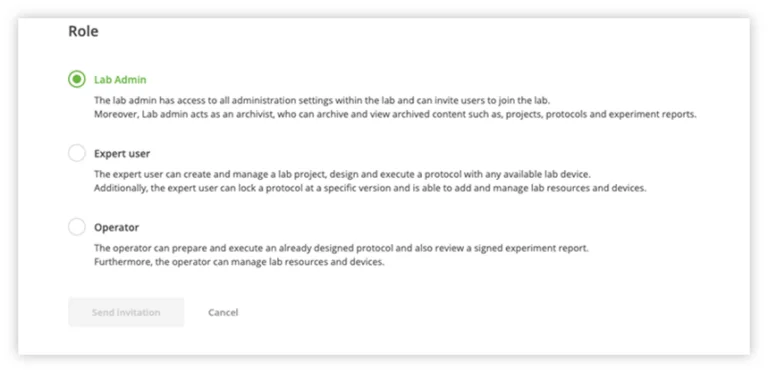
OneLab includes three user roles with a hierarchy of privileges. Each of these roles (Operator, Expert and Administrator) and how they contribute to lab traceability will be explored in this blog series.
Selecting and starting a predefined protocol
Once a defined experimental protocol is available, any analyst logged in as an Operator or Expert and with access to the designated lab workspace where data are shared, can follow this protocol to perform laboratory experiments. The Operator role cannot modify a protocol at all, so there is additional assurance that it will be followed precisely. In addition, the capability to lock a protocol will also deter an Expert user from making changes to a locked protocol.
For a fully automated experiment in OneLab, the analyst will prepare the correct devices, tools and reagents required. Once the protocol is designed, the instrument is set up, and the samples are ready, the Andrew+ robot can automatically execute the protocol.
For manual execution of a protocol, the analyst will prepare the equipment and solutions, and then follow the protocol execution instructions step by step, as instructed. As each step is performed, clear text and graphical instructions will guide the analyst, and critical information will be recorded for the duration of the protocol.
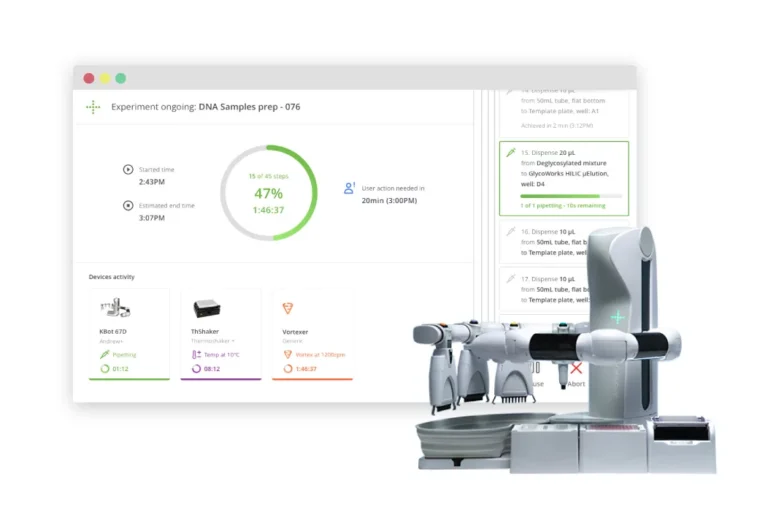
Recoding the protocol execution in an Experiment Report
All analyst (or robot) actions are recorded in the experiment, in an audit trail-like experiment log, with an indication of elapsed time from the start of the experiment. OneLab records accurate timestamps and information about the duration of each specific step. This can be a useful traceability tool to determine if a step was performed accurately and correctly in an appropriate or expected amount of time.

Releasing, reviewing, and approving Experimental Reports
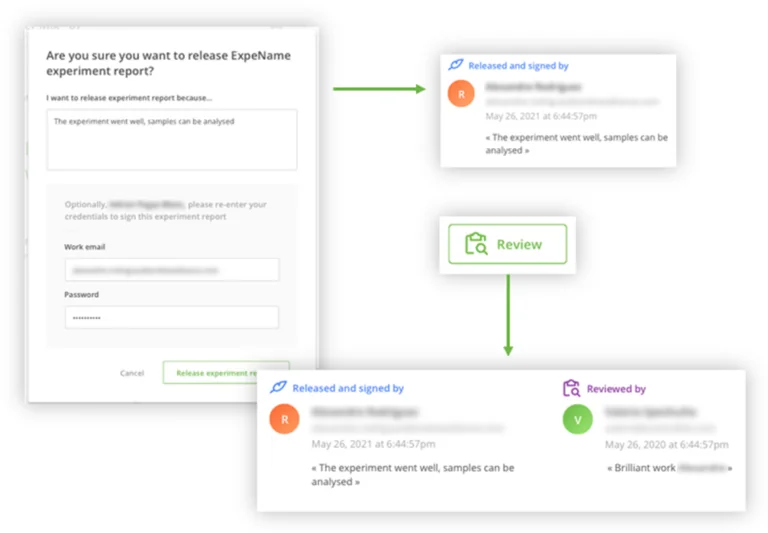
Once an experiment is completed, the analyst may simply look over and release the Experiment Report. This frees up any devices or equipment used so that other analysts can use it for their own work. Releasing a report is audit trailed.
However, in regulated laboratories, it may be required for the analyst who completed the task, to review and electronically sign the Experiment Report. This is performed by re-entering the username and password, along with a conclusion about the review, or a reason for the review.
The action of applying an electronic signature to an Experiment Report enables a second person to review and/or approve the document. While the person who reviewed the document may or may not be the same user that executed the experiment, rules in OneLab will prevent the analyst who performed the task from approving their own work.
These actions are recorded in the audit trail for this specific report, but also as part of the overall Lab Audit Trail.

Documenting errors and repeats
OneLab can help a user document errors and mistakes because not all experiment completes perfectly each time. If any experiment step has an error, it will always be recorded in OneLab. This error recording is expected with an automated system but is also important when an analyst is performing a manual execution. When encountering an error (such as ’tip cannot be inserted’ or ‘collision with an obstacle’), the Andrew+ instrument stops completely, allowing for a user to remediate the error. Depending on the gravity of the error, the experiment can then continue from where it left off or, alternatively, OneLab may force the analyst to restart the entire experiment. If an analyst makes a mistake during a manual experiment execution or with the Pipette+ guided pipetting device (like dispensing an incorrect volume or pipetting in the wrong well or tube), the analyst has the ability to document their error with an explanation and a remediation step.
Regardless of the errors encountered, they (and any incomplete steps) will be readable and traceable by the reviewer of the experiment in the experiment log report easily found within OneLab.
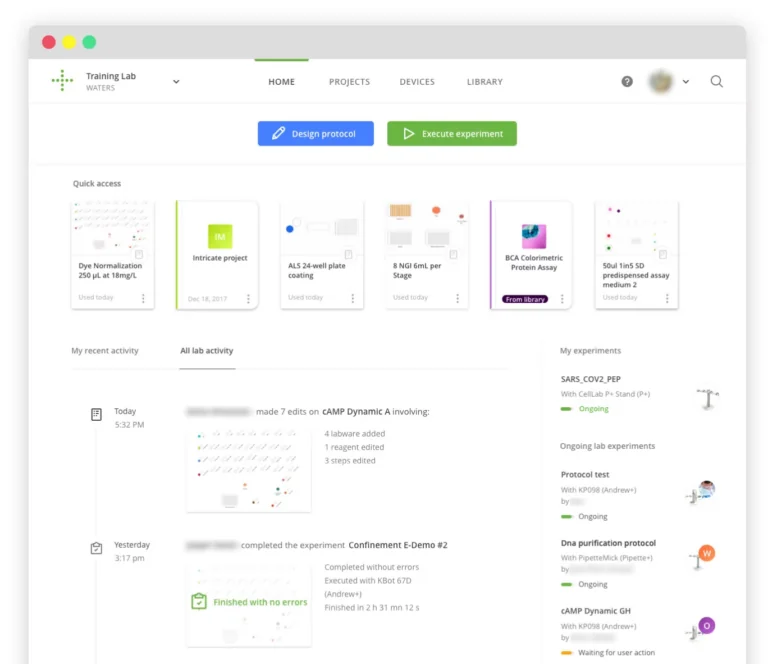
Tracking ongoing work
An analyst might not be able to complete an experiment in one session. Upon login, OneLab allows an analyst to view all their own experiments that are in progress and those that were recently completed.
The traceability provided by OneLab allows a laboratory manager to view all work completed or in progress, across their entire lab, through the OneLab Workspace. Managers can also be confident that every experiment will have the traceability to all devices and tool logbooks used for the task.
Conclusion
When automating the execution and/or documentation of lab methods to improve quality and integrity of data, two approaches can be taken:
- Automate as much as possible, so that a process or experiment can be validated one time, ensuring confidence that it will be completed the same way each time it is executed. This is the path to true “review by exception” where only experiments or activities where any failures, deviations or exceptions are flagged and need to be reviewed.
- Guide the analyst through the procedure, ensuring that they can only operate within the defined parameters of the process while documenting, as automatically as possible, all the analyst’s actions, thereby simplifying the second person review process.

With OneLab, both approaches are possible; either guiding a logged-in analyst to manually execute a predesigned protocol while prompting them to document any additional actions contemporaneously, or utilize the Andrew+ robot to fully automate the protocol while still documenting every step taken. In both cases, OneLab automatically controls and tracks any connected device that is used in the experiment for optimal traceability.
Learn how an Expert user designs a protocol in OneLab and how a Lab Administrator configures user access, device inventories, and takes care of historical records in the following blogs.
Related content:
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (22) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (20) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)