Atlantis dC18 Columns are a fully LC-MS compatible line of reversed-phased (RP) columns designed for retaining and separating both polar and non-polar compounds. This new silica based packing material has excellent stability in acidic mobile phases and is fully compatible with 100% aqueous mobile phases. With Atlantis dC18 Columns, direct scale-up from analytical to preparative dimensions is simple. To demonstrate the utility of Atlantis dC18 Columns, we separated and purified three mixtures of polar compounds. Our results indicate that Atlantis dC18 Preparative Columns provide excellent efficiency, high mass loading, and ease of scale-up.
Chromatographers have often struggled with retaining polar compounds on traditional RP columns (e.g., C18 stationary phase). These columns are too hydrophobic to retain the polar analytes. Additionally, these traditional columns often dewet under the 100% aqueous conditions necessary for retaining these polar analytes. The Atlantis dC18 material was designed with the best combination of pore size, ligand density and ligand type to retain polar analytes and to operate in 100% aqueous conditions without dewetting. We have developed separations of polar mixtures on analytical dimension Atlantis dC18 Columns. We then scaled-up the separations to preparative dimensions to demonstrate the utility of these columns for compound purification.
Analytical separations were performed on an Atlantis dC18, 4.6 x 100 mm, 5 μm Column. Preparative separations were performed on an Atlantis dC18, 19 x 100 mm, 5 μm Column. All experiments were conducted at ambient temperatures. The sample in Figure 1 is a mixture of 1 mg/mL each of sulphanilamide, sulphathiazole, sulphamethazine and sulphamethoxazale prepared in deionized (DI) water. The sample in Figure 2 is a mixture of pyrodoxal, folic acid and caffeine at 1, 0.25 and 1 mg/mL, respectively, in DI water with 0.2% ammonia to increase the solubility of folic acid. The sample in Figure 3 is a mixture of cinoxacin, oxolinic acid and nalidixic acid at 5, 0.5 and 1 mg/mL, respectively, dissolved in DMSO. The mobile phase consisted of A: water with 0.1% formic acid, and B: acetonitrile/water (90/10) with 0.1% formic acid. 0.1% TFA was used in place of formic acid for the separation in Figure 2. Gradient methodology for each separation example is listed in each figure caption. The flow rate was 1.0 mL/min for the 4.6 mm i.d. column and 17.06 mL/min for the 19 mm I.D. column. All experiments were run on the Waters AutoPurification System, which consists of a Waters 2525 Binary Gradient Module, a Waters 2767 Sample Manager, a Waters 2996 Photodiode Array Detector and a Waters ZQ Mass Spectrometer.
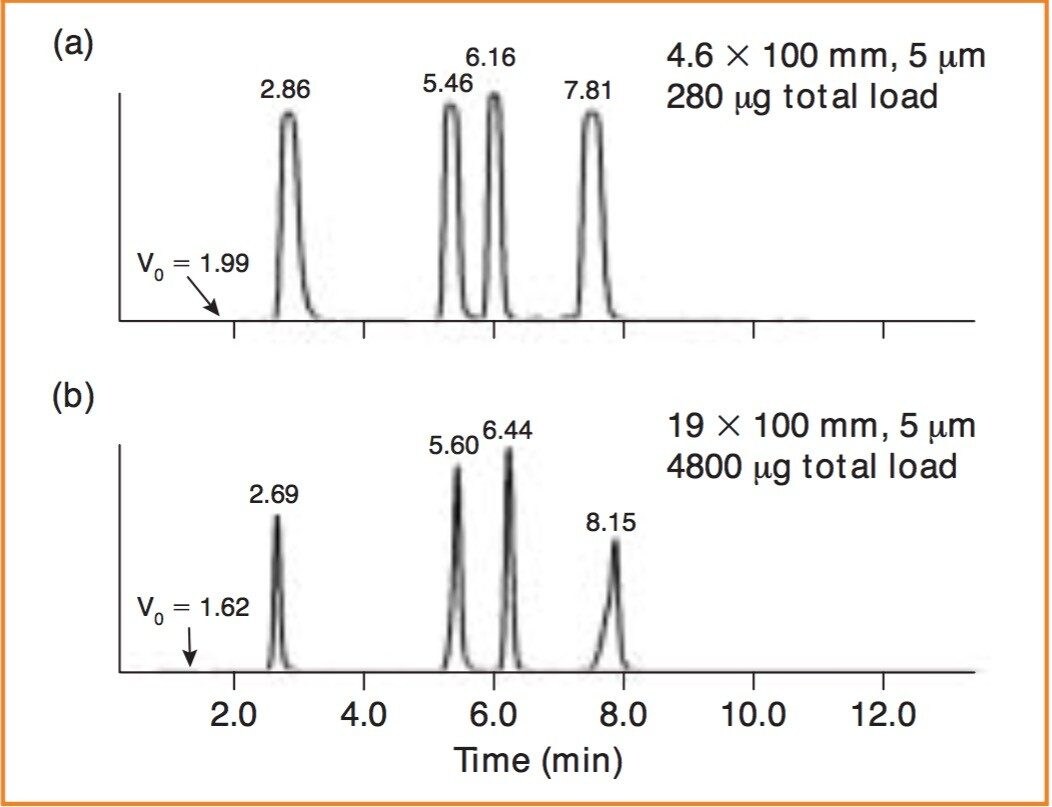
The retention and separation of the sulphonamides on the analytical column is shown in Figure 1A. The total load is 280 μg and the flattened profiles reflect the saturation of the PDA detector. The mass load was proportionally scaled-up and run on the preparative column as shown in Figure 1B. Note the direct scale-up, excellent peak shapes and total mass load of 4800 μg.
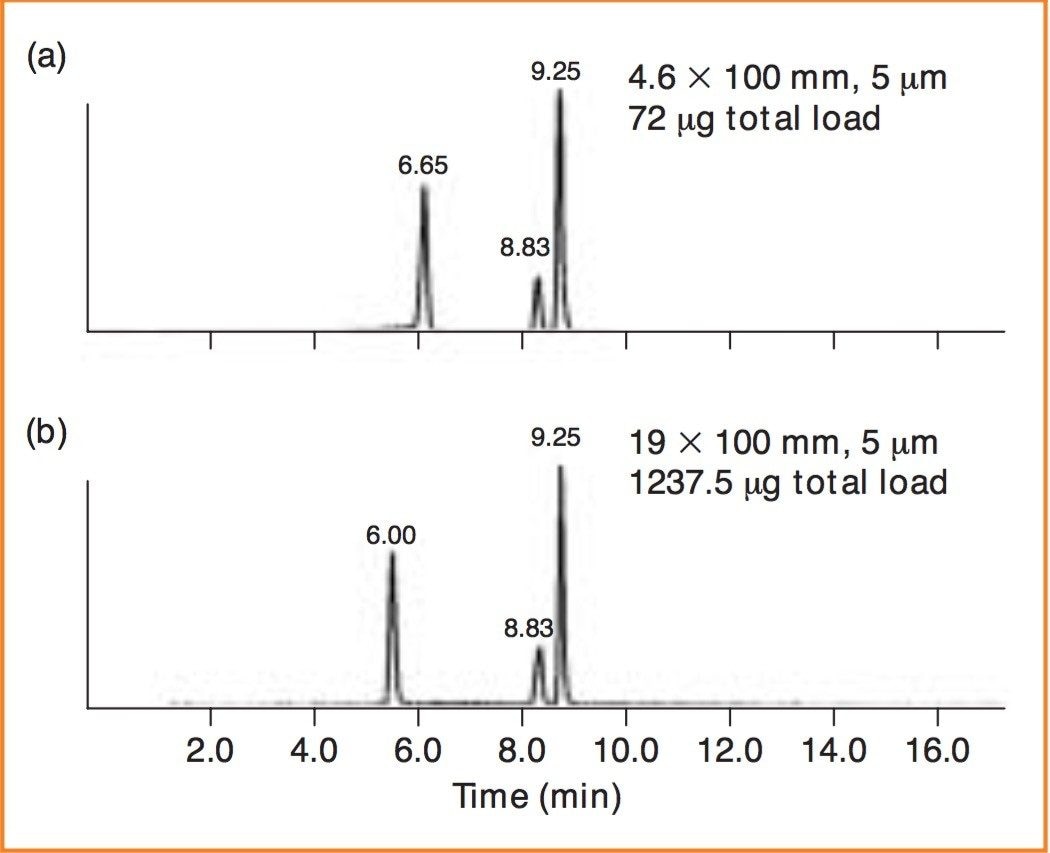
The retention and separation of pyrodoxal, folic acid and caffeine on the analytical column is shown in Figure 2A. The total load in this experiment is 72 μg. The mass load was proportionally scaled-up and run on the preparative column as shown in Figure 2B. Again, note the direct scale up, excellent peak shapes and total mass load of 1237.5 μg.
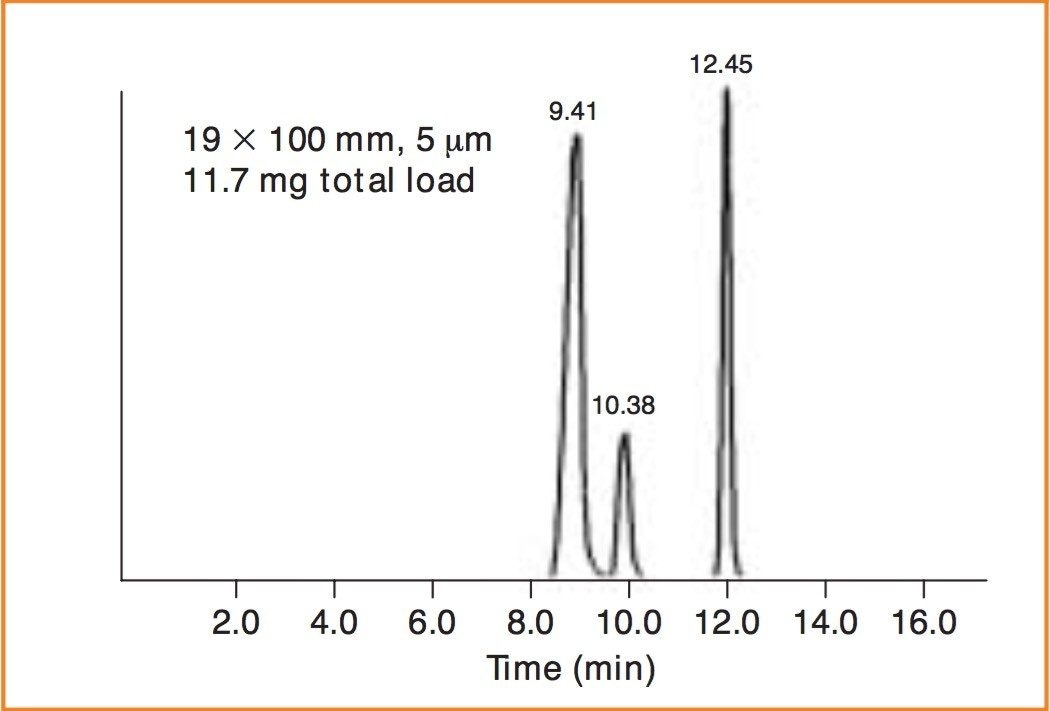
The final mixture was run directly on the preparative column and is shown in Figure 3. For this set of analytes, we achieved a total load of 11.7 mg.
Atlantis dC18 RPLC Columns are useful tools for retaining and separating polar compounds under highly aqueous mobile phase conditions. Since Atlantis dC18 Columns are fully LC–MS compatible, they can be used in a mass-directed chromatographic purification system. Atlantis dC18 Preparative Columns provide excellent efficiency, high mass loading, and ease of scale-up.
720000742, September 2003