
This work demonstrates the advantages of using gas chromatography with tandem quadrupole MS/MS detection in the analysis of growth promoter residues in animal products. Comparison is made with the traditional single quadrupole and high resolution sector GC-MS techniques widely used in this field. Examples are shown covering several classes of banned substances.
The performance of tandem quadrupole MS/MS in terms of sensitivity and specificity permits some measurements at trace levels that could not previously be achieved. As a consequence, the decision limits (CCα) obtained by these new methods are lower, thus, the control of the abuse of forbidden substances is clearly improved.
Moreover, tandem quadrupole MS/MS considerably increases confidence in the unambiguous identification of the target compounds, in accordance to the criteria fixed by the 2002/657/EC decision.1
It should be noted, however, that for these advantages in MS/MS measurement to be realized, sample preparation remains a critical procedure to reduce matrix effects such as coelutions, crosstalk effects, or ion suppression phenomenon. Also, the efficiency of any derivatization reaction must also be considered if performance is to be maximized.
Details of the sample preparation procedures used in this work are beyond the scope of this paper and can be found elsewhere.2-5
For the maximum confidence and confirmation, the use of GC-MS/MS is now a clear requirement for the laboratory analyst
A wide range of drugs including hormones, antibiotics, and antihelmintics are used in farm animals for therapeutic purposes. Some of these drugs have also been used by some farmers to alter the characteristics of the meat in cattle by improving growth performances. In 1985, the use of compounds with hormonal or thyreostatic action was banned within the EU unless under veterinary prescription. European countries have implemented screening programs to reduce the abuse of such drugs and to ensure that meat sold for human consumption was free from residues. Policing of the illicit use is carried out under EU directive 96/23/EC and programmed under the National Plan of individual member states.
Whilst there is intensive control of illegal growth promoters within the EU Member States, a number of reported positive samples in cattle for substances with estrogenic, androgenic or gestagenic effect, ß-agonists, corticosteroids, and thyreostats have been reported in recent years. In a comparative study conducted in the European Union in 2000, monitoring showed positive results for these substances in cattle and pigs in six countries. Five Member States reported positive findings for bovine meat and three countries reported positive samples for porcine meat.
Concentration levels are ever decreasing, and steroid misuse appears likely to shift toward the use of natural hormones. This means that traditional methods of detection will need to be improved in order to maintain efficient control of such substances within the food chain.
For GC-MS experiments, a single quadrupole mass spectrometer (low resolution single MS), and a double focusing electromagnetic instrument (high resolution MS) were used. For the GC-tandem quadrupole MS/MS experiments, a Micromass VG Quattro II Mass Spectrometer and a Waters Micromass Quattro micro GC Mass Spectrometer were used.
Injector and transfer line temperatures were set at 250 °C and 280 °C, respectively. Source and analyzer temperatures were set to 280 °C and 100 °C, correspondingly. The capillary column was OV-1 (Ohio-Valley) 30 m x 0.25 mm i.d., with a film thickness of 0.25 μm. The following temperature program was used: 120 °C (2 min), then 15 °C min-1 to 250 °C (0 min), then 5 °C min-1 until 300 °C (10 min). Helium was used as carrier gas at 1 mL/min-1. Electron impact (EI) was set at 70 eV in all cases.
Diethylstilbestrol was analyzed initially by GC-MS, where confirmation using low resolution single quadrupole selected ion recording (SIR) would require a minimum of four ions to be acquired. The sample extracts were derivatized with TMS prior to injection. A blank urine sample was analyzed, followed by a spiked urine sample, spiked at 1.5 ppb. Figure 1 shows traces for the blank urine, and Figure 2 shows traces for the 1.5 ppb spiked urine.
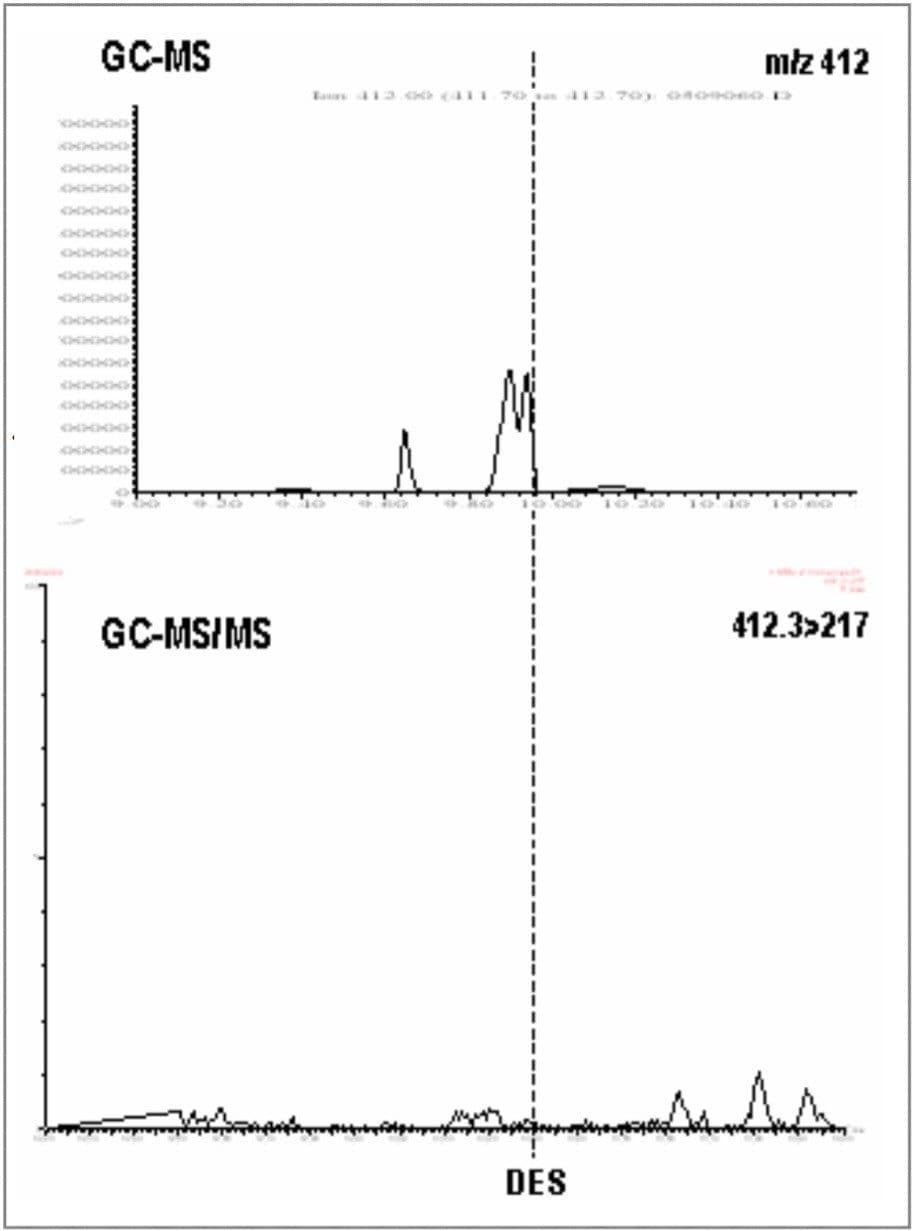
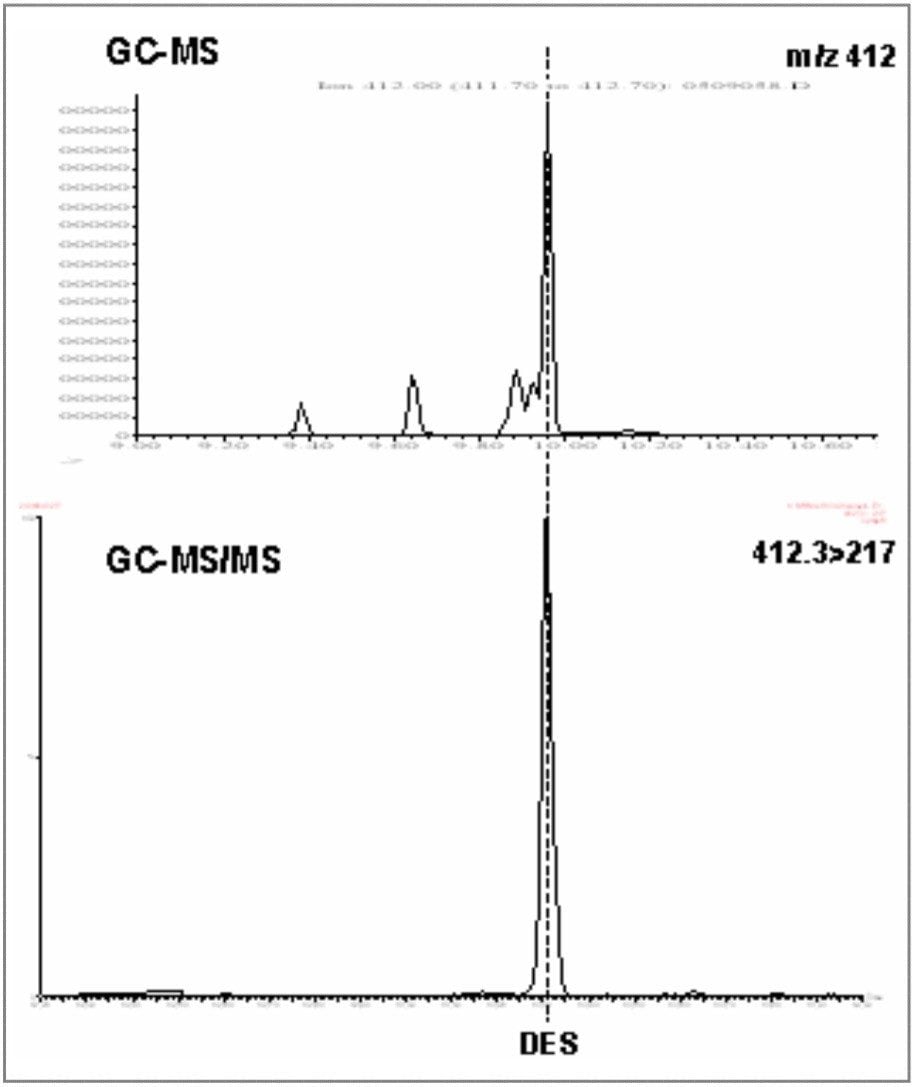
If the results of the GC-MS analysis are compared with the same extracts acquired by GC tandem quadrupole MS/MS monitoring only two MRM transitions (thus the required four EU identification points), it is clear that unambiguous identification and confirmation is achieved with much better signal-to-noise ratios for the target peaks in the spiked urine, and a much cleaner blank in the retention time region of interest.
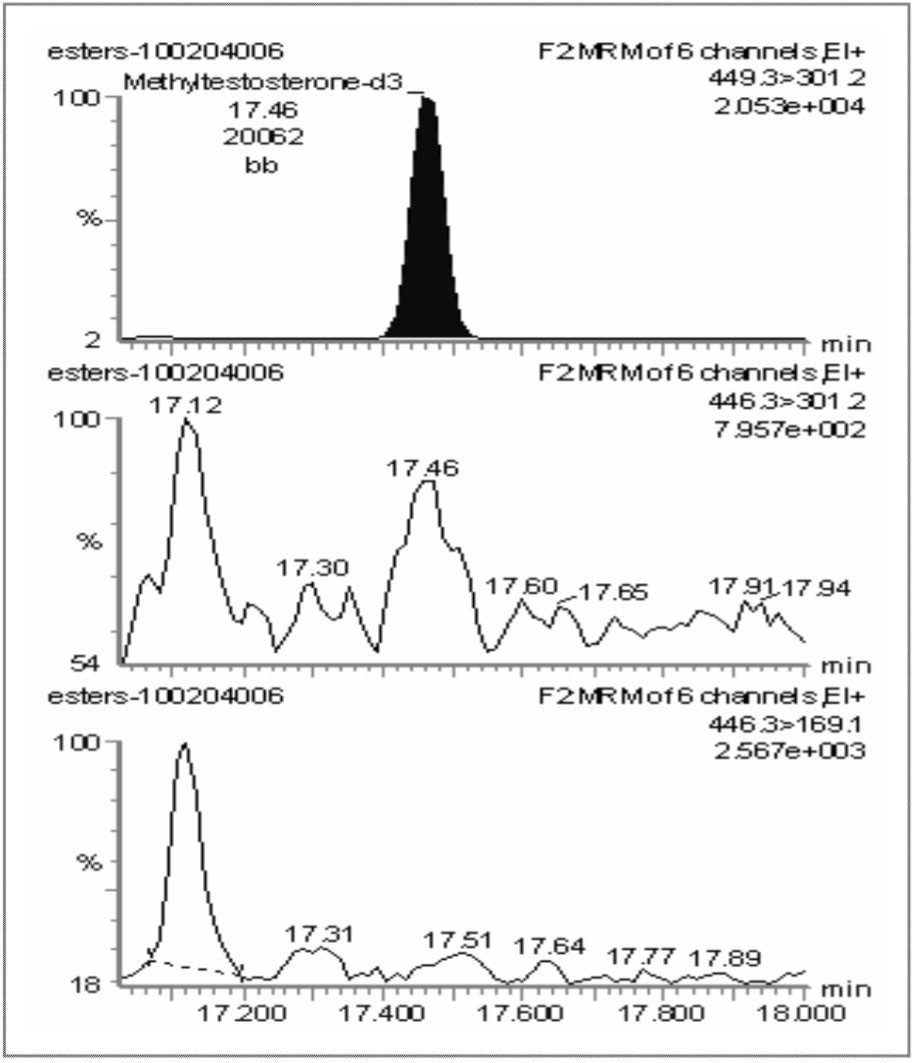
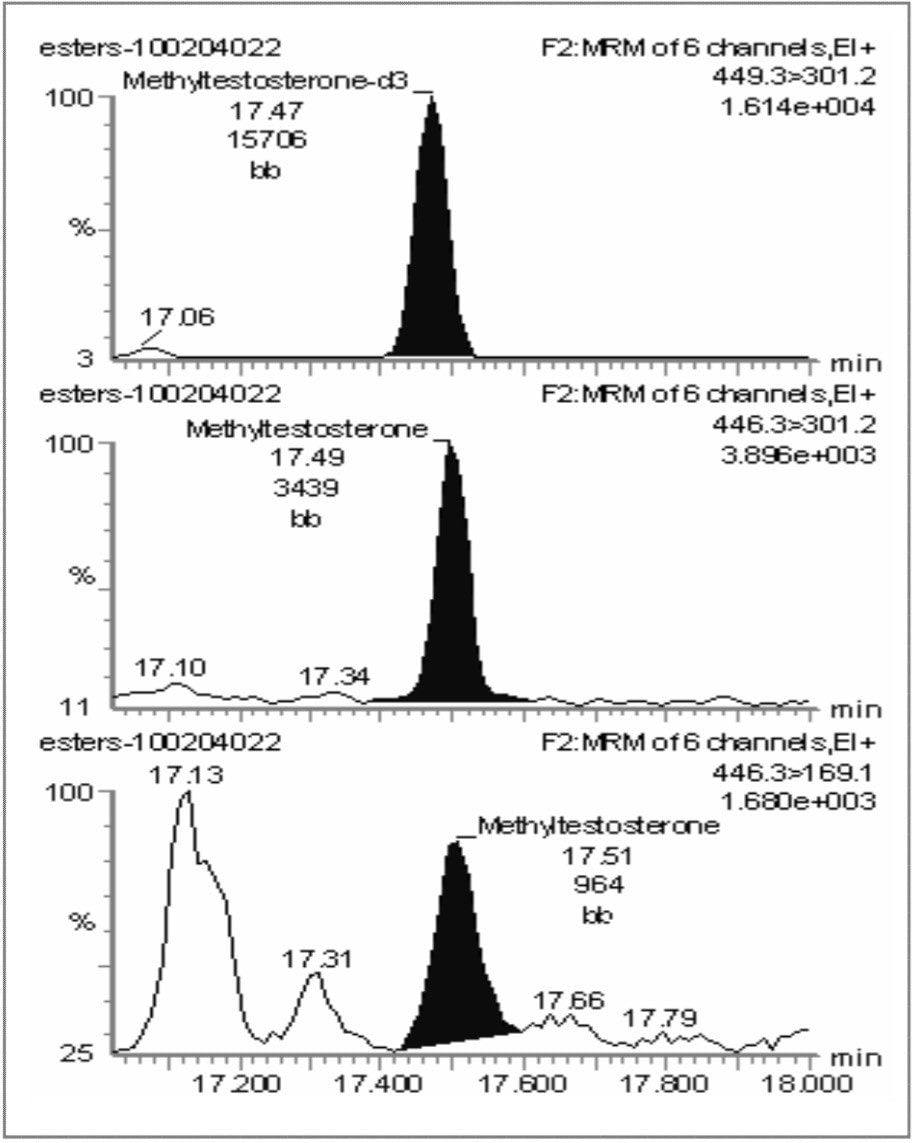
Hair samples were taken from an animal immediately prior to administration of methyl testosterone (D0) to yield a blank sample, followed by further sampling 84 days after administration (D84). Two MRM transitions were monitored for methyl testosterone and one for the d3-labeled methyl testosterone internal standard. Figure 3 presents the chromatograms for the D0 blank sample, and Figure 4 presents the chromatograms for the D84 incurred hair sample.
It can be seen that 84 days after administration, methyl testosterone can still be easily detected and confirmed by the use of GC-MS/MS.
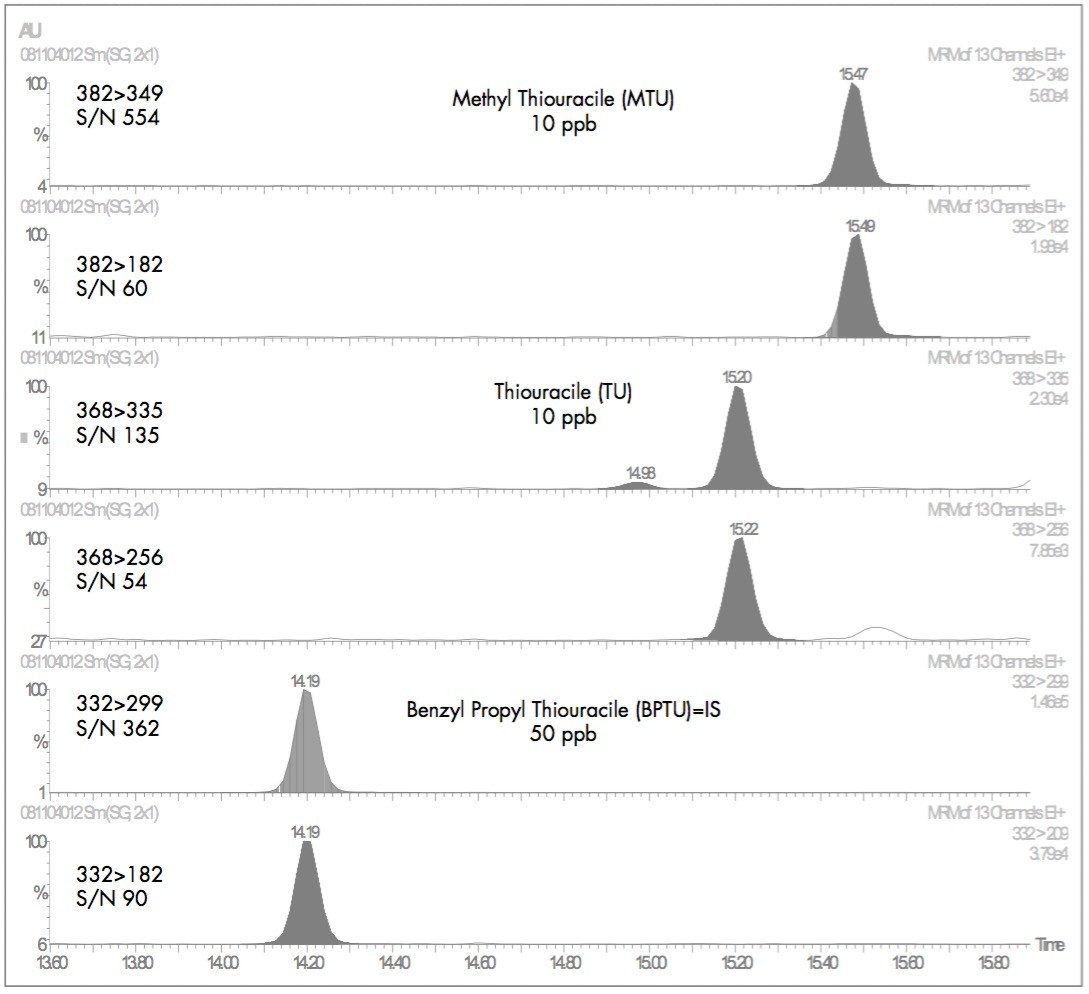
Thyreostatspose a particular challenge to the analyst as they require careful derivatization prior to LC or GC analysis. Using a specific derivatization method which prevents the formation of tautomeric forms, the compounds were successfully analyzed by GC tandem quadrupole MS/MS. Figure 5 presents the data for two thyreostats at 10 ppb in urine, with an internal standard at a level of 50 ppb.
It can be seen that the peaks are all well resolved from any background peaks, allowing clear determination and confirmation of the target analytes. The MRM transitions with relevant signal-to-noise ratios for both the quantification and confirmation transitions are shown in Figure 5.
At a level of 10 ppb in urine, excellent sensitivity is maintained for the weaker confirmatory MRM transitions with the lowest signal-to-noise, for a confirmatory transition of 54:1 for thiouracile.
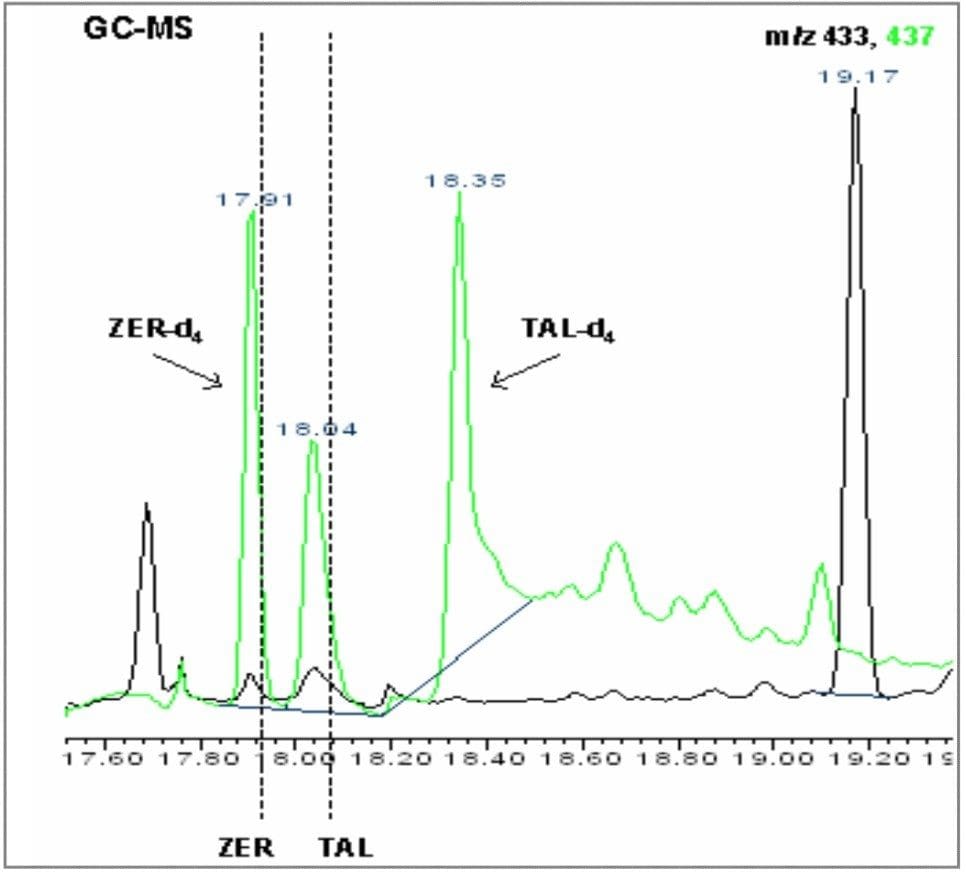
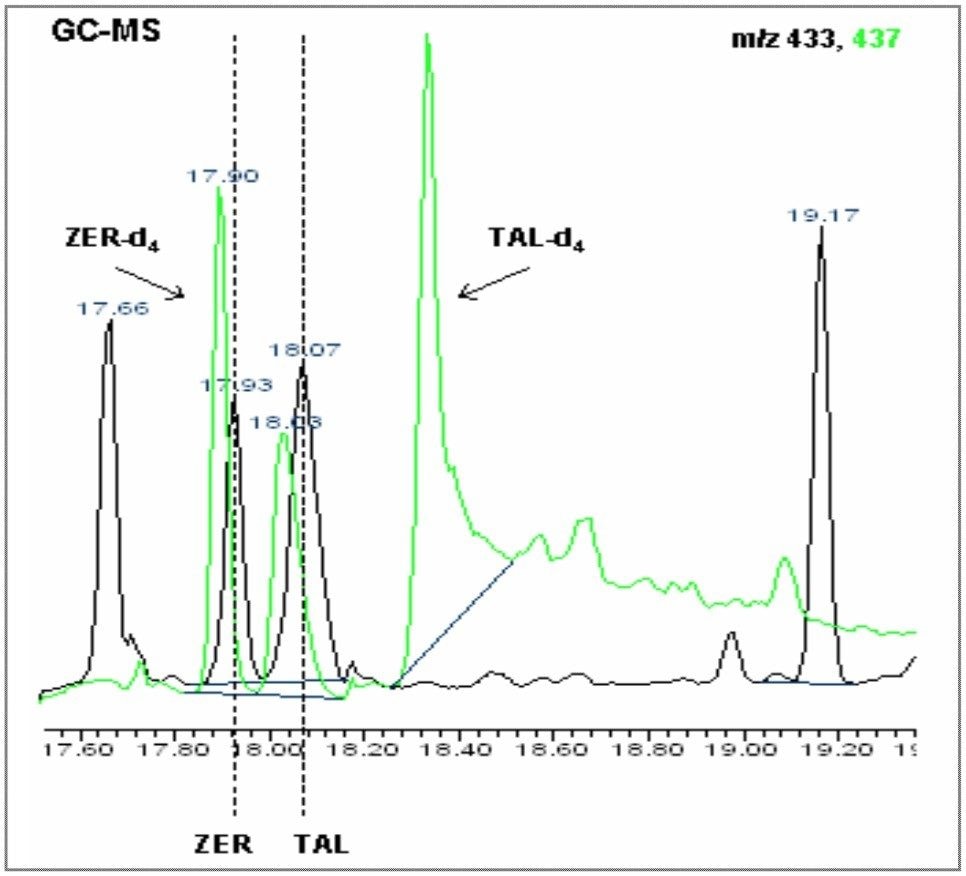
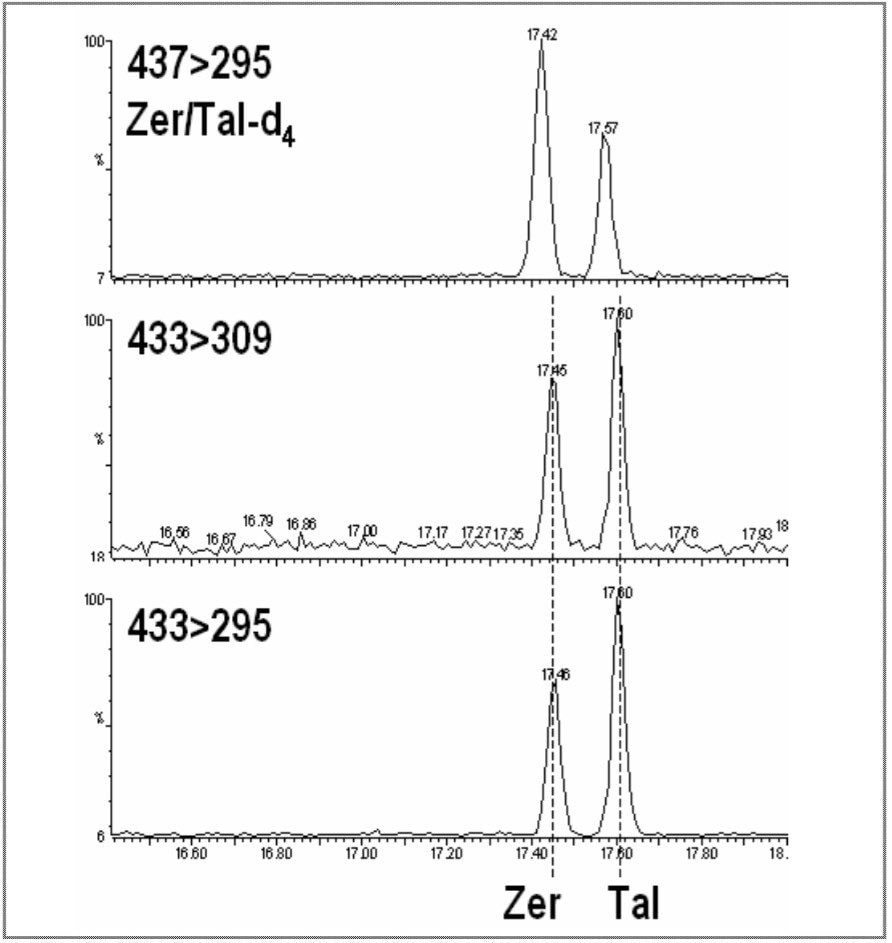
A blank and a spiked hair sample were analyzed by GC-MS (SIR) and then by GC-MS/MS. The GC-MS blank shows elevated noise, causing a reduction in the method LOD/LOQ, whilst with the spiked hair sample, the target compounds zeranol (ZER) and taleranol (TAL) can be clearly detected, but not confirmed. Figures 6 and 7 show the GC-MS chromatograms for the blank and spiked hair samples.
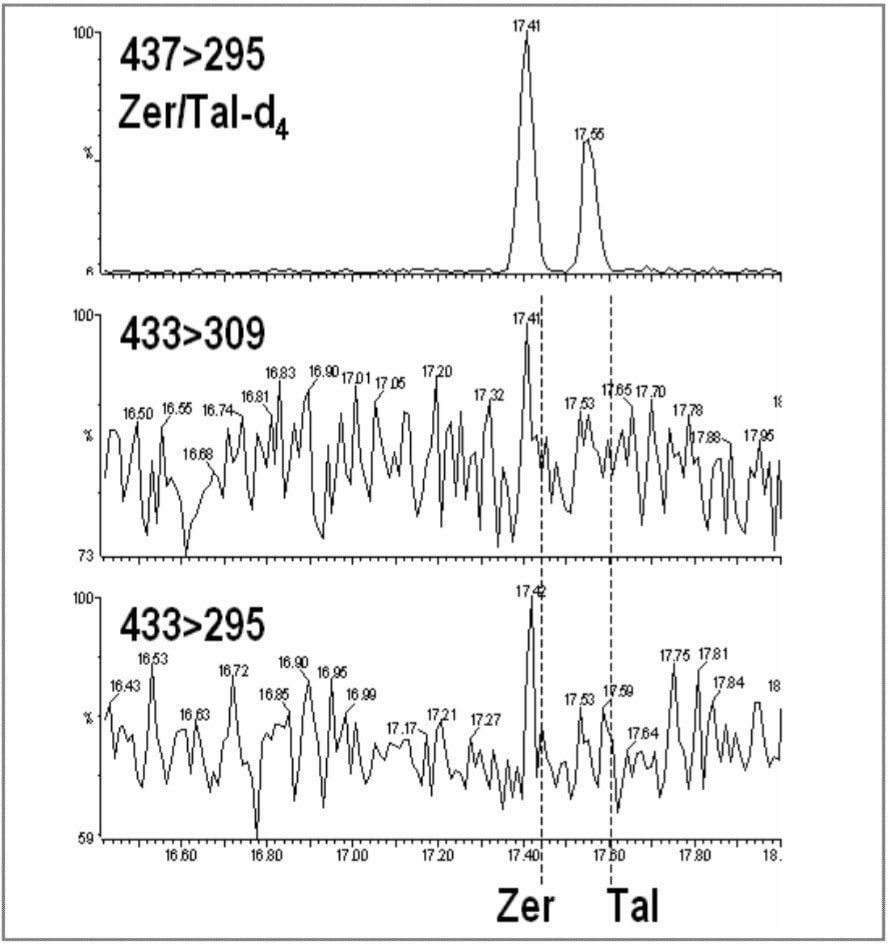
Figures 8 and 9 show the tandem quadrupole GC-MS/MS chromatograms for the blank and spiked hair samples. The blank traces do not show any significant interference, with good specificity for the monitored signals. The spiked hair sample injection shows identification of the target analytes and internal standards with two MRM transitions, thus achieving the EU four point requirement for identification.
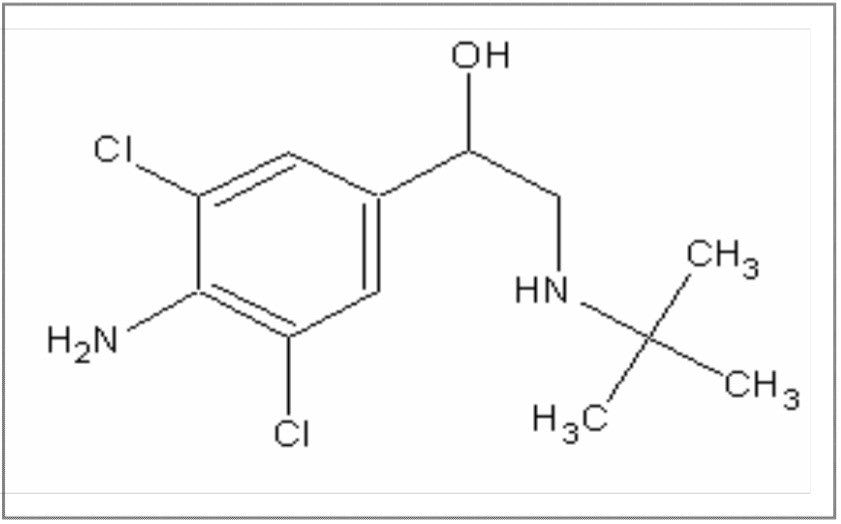
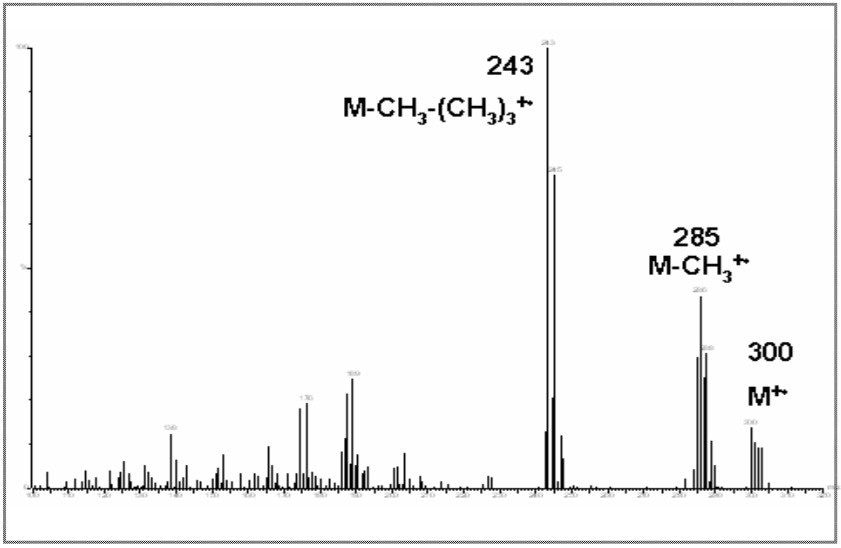
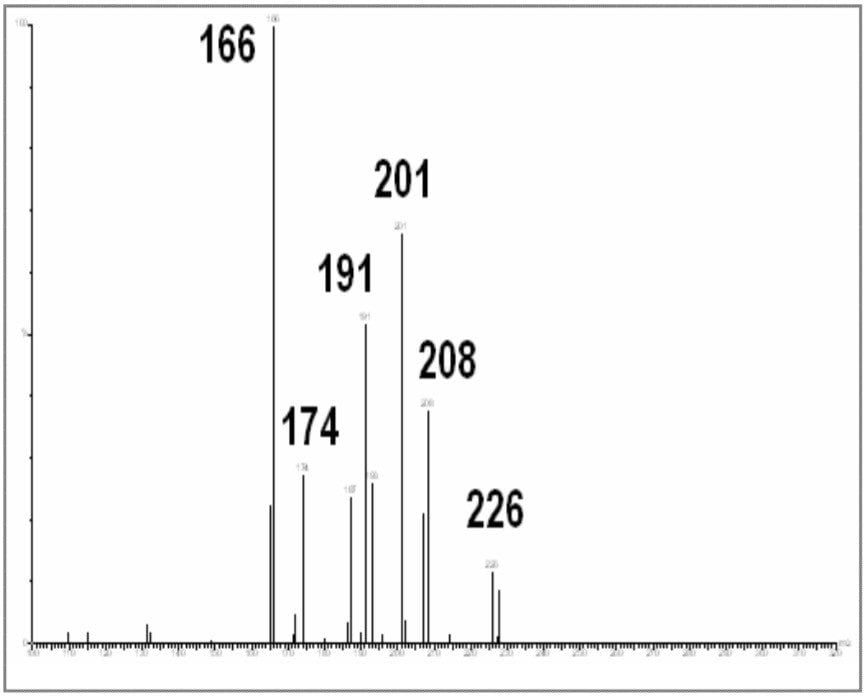
Clenbuterol (Figure 10) requires derivatization prior to GC analysis due the number of polar functional groups on the molecule. The boronic derivative (Figure 11) is more amenable to GC separation. The full scan mass spectrum (Figure 12) of the derivative displays a weak molecular ion at m/z = 300, with only two diagnostic ions, which have medium specificity. This would make confirmation by GC-MS difficult.
Selecting the base peak (m/z = 243) from the full scan mass spectrum, and submitting it for product scanning produces very helpful and specific fragmentation. The product scanning spectrum for m/z = 243 is shown in Figure 13 shows specific and selective product ion at masses 166 and 191.
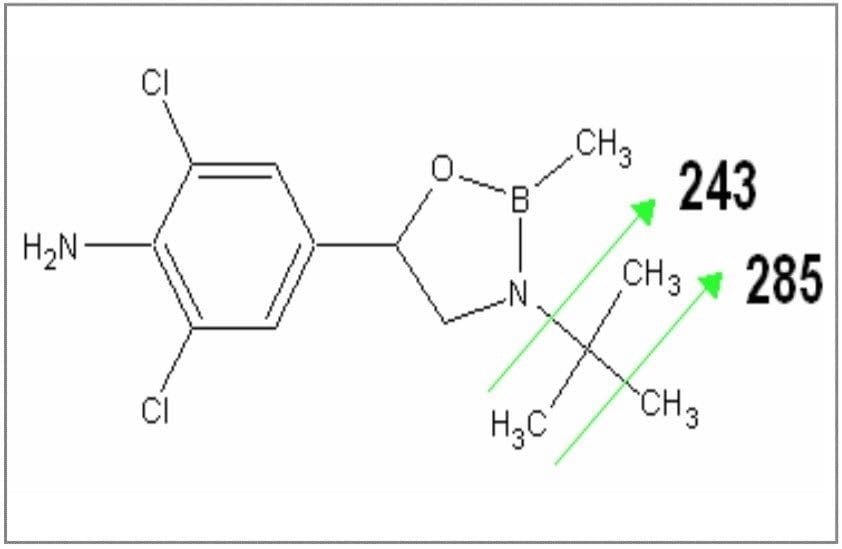
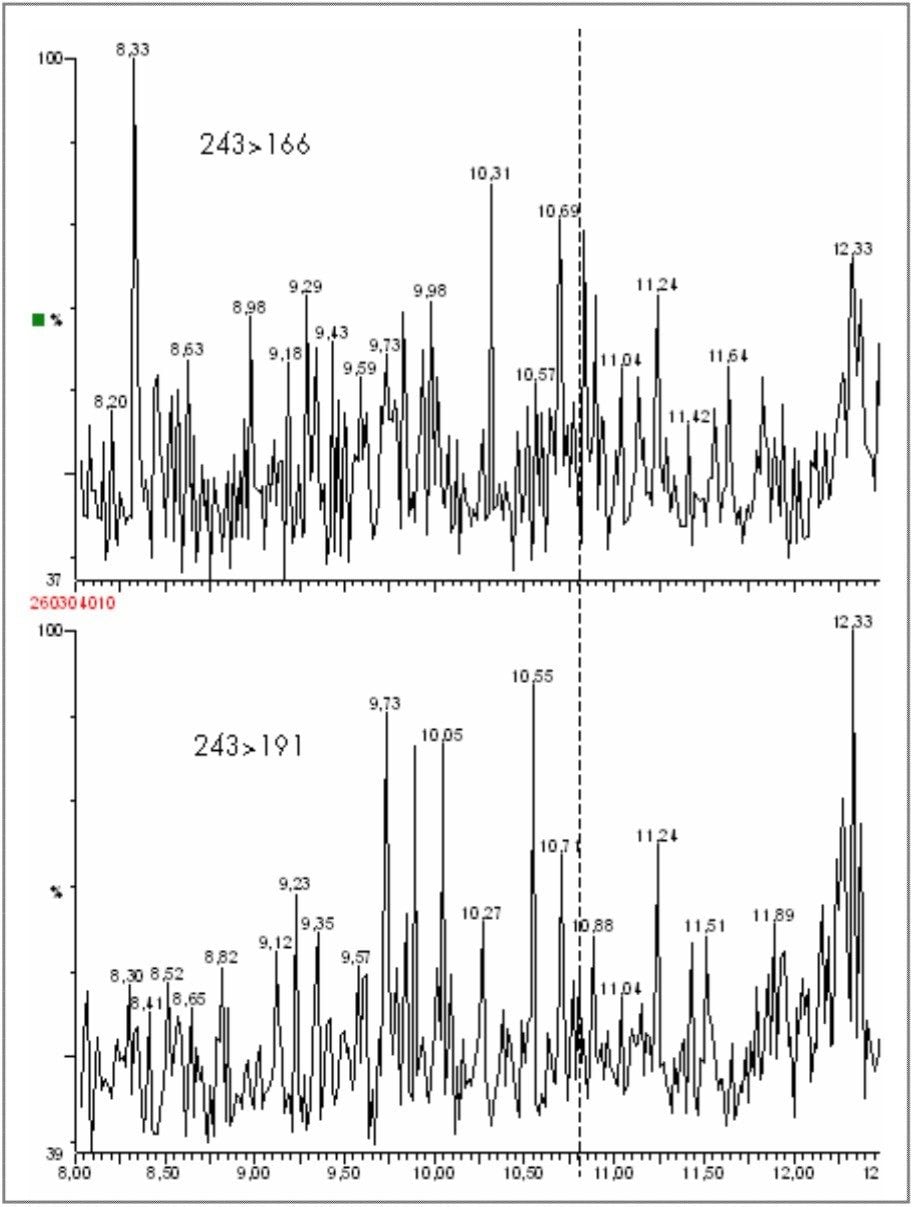
From the product scanning of m/z = 243, the MRM transitions 243>166 and 243>191 were selected for confirmatory analysis. Analysis of a urine blank confirmed the high specificity of these MRM transitions, with no interferences observed in the region of interest for the boronic derivative of clenbuterol. Figure 14 shows the chromatogram for the urine blank acquired in MRM mode.
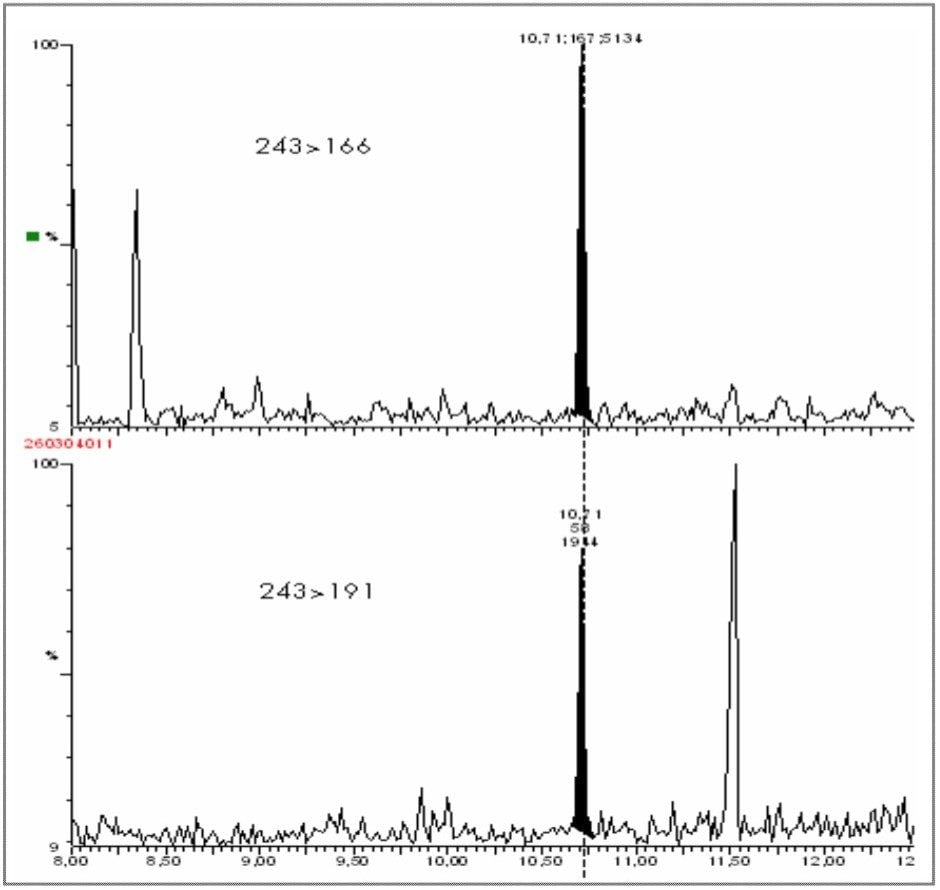
Aurine sample was then spiked at a level of 40 ppt with clenbuterol, and analyzed by GC-MS/MS in MRM mode. Figure 15 shows the chromatograms obtained, demonstrating clear confirmation of clenbuterol with impressive sensitivity.
720001124, February 2005