For research use only. Not for use in diagnostic procedures.
This aplication note presents the application of HILIC for the analysis of monoamine neurotransmitters using an XBridge BEH Amide XP column. This method achieves baseline separation for the most polar and challenging analytes in a short analysis time without the use of ion-pairing reagents that are often necessary in reversed-phase analysis of these compounds.
Hydrophilic-interaction chromatography (HILIC) is increasingly becoming a method of choice for the analysis of polar compounds [1-5]. One set of polar analytes that poses particular challenges are the monoamine neurotransmitters, dopamine (DA), serotonin (5-HT), epinephrine (EP), and norepinephrine (NE). These compounds play a significant role in many mood, movement, and other neurological disorders such as depression, anxiety, schizophrenia, and Parkinson’s disease[6-8]. These neurotransmitters also play a critical role in the effects and toxicity of drugs of abuse[9-11].
This work presents the application of HILIC for the analysis of monoamine neurotransmitters using an XBridge BEH Amide XP column. Development of the successful chromatographic method depends upon the systematic optimization of organic mobile phase ionic strength to obtain the best peak shape and sensitivity. Choice of stationary phase and column temperature are also important. The resulting method achieves baseline separation for the most polar and challenging analytes in a short analysis time without the use of ion-pairing reagents that are often necessary in reversed-phase analysis of these compounds.
Combined stock standards of dopamine, norepenephrine, epinephrine, serotonin, and N-methyl serotonin (NMS) were prepared in methanol containing 0.1% ascorbic acid and 2.5% 1N HCl to prevent oxidation. Working standards of 100 ng/mL DA, NE, EP, 5-HT, and 10 ng/mL NMS were prepared fresh each day in starting mobile phase conditions.
|
LC System: |
ACQUITY UPLC |
|
|
Column: |
XBridge BEH Amide XP, 2.5 μm; 2.1 x 75 mm. P/N 186006090 |
|
|
Column Temp: |
30 °C |
|
|
Sample Temp: |
5 °C |
|
|
Mobile Phase A: |
95:5 water:acetonitrile containing 100 mM NH4HCOO, pH 3.0 |
|
|
Mobile Phase B: |
85:15 acetonitrile:water containing 30 mM NH4HCOO, pH 3.0 |
|
|
Needle Washes: |
Strong and weak needle washes were both placed in MPB |
|
MS System: |
Xevo TQ-S |
|
Ionization mode: |
ESI Positive |
|
Acquisition mode: |
MRM |
|
Capillary Voltage: |
2.0 kV |
|
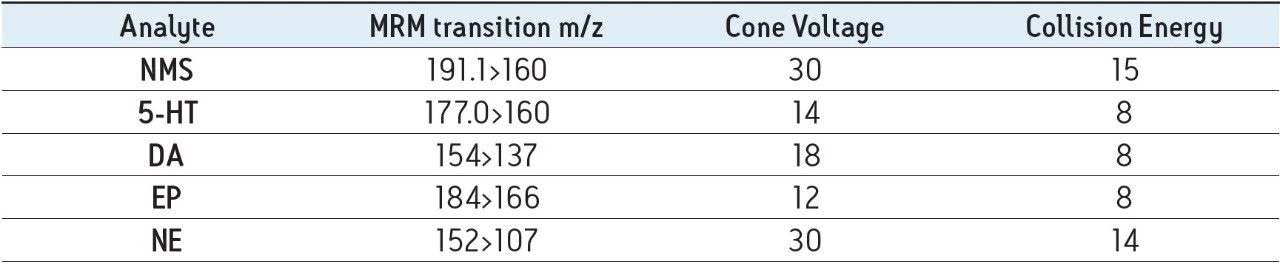
Cone voltage: |
Compound specific (see Table 1) |
|
Desolvation Gas: |
900 L/hr |
|
Cone Gas: |
150 L/hr |
|
Desolvation Temp: |
350 °C |
|
Source Temp: |
100 °C |
Data was acquired and analyzed using MassLynx Software (V4.1; SCN 810).
Initial mobile phase conditions were 100% MPB. The percentage of MPA was increased to 30% over 2.5 min. The percentage of MPB was returned to 100% over 0.1 minute and held there for 1.4 min. The total cycle time was 4.0 min. The injection volume was 20 μL.
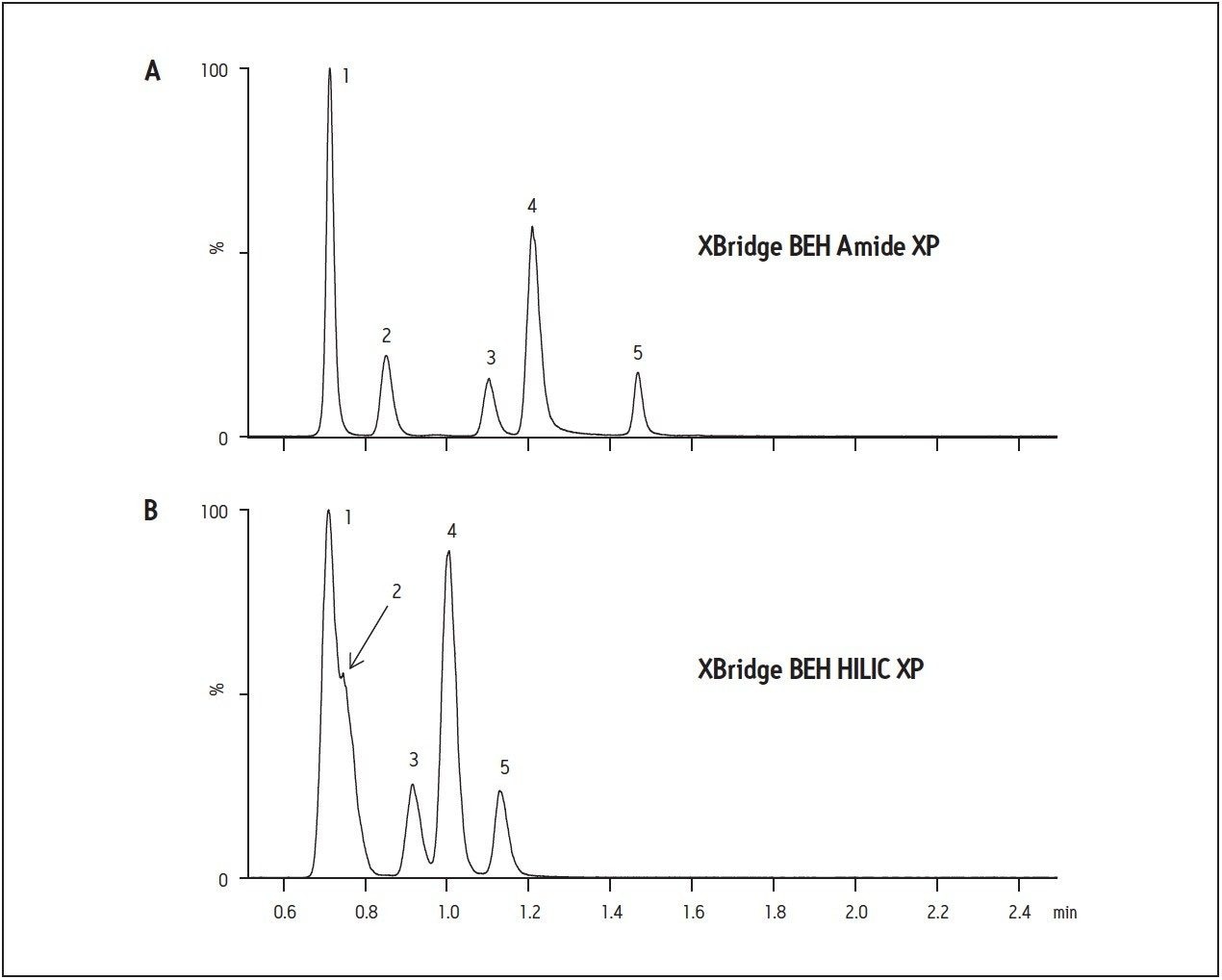
Figure 1A shows the chromatography of monoamine neurotransmitters analyzed on the XBridge BEH Amide XP column. In order to achieve acceptable peak shape and resolution, it was necessary to carefully balance the ionic strength of MPB with solubility. 30 mM NH4HCOO in MPB was required to achieve the chromatographic performance seen in Figure 1. The use of mobile phases with lesser ionic strength (10 and 20 mM) resulted in significant peak tailing and poor resolution between DA, EP, and NE, the most polar analytes, possibly due to secondary interactions with the stationary phase itself. Increasing the aqueous content of MPB to 15% ensured complete miscibility and prevented phase separation between water and acetonitrile that occurred at lower aqueous concentrations. Increasing the aqueous content alone resulted in a predictable decrease in retention for all compounds, but did not result in any improvements in peak shape or resolution between adjacent peaks. These changes are all consistent with the theory that increasing mobile phase ionic strength can disrupt secondary interactions with the stationary phase, resulting in improved chromatography.
The performance of the XBridge BEH Amide XP column detailed above was compared to the unbonded hybrid particle (XBridge BEH HILIC XP). Preliminary work with 10 mM ammonium formate in MPA and MPB had shown that the compounds in this study exhibited better separation and resolution on the Amide column vs. the XBridge HILIC column. Comparison of the two columns using the optimized conditions described above confirmed those initial results. Figure 1B shows chromatograms of monoamine standards analyzed on the XBridge BEH HILIC XP column. Clearly, retention of nearly all analytes is superior on the Amide column as is the resolution between adjacent peaks. The superior performance of the Amide column may be attributable to its polar functional group. In an acidic environment (pH 3.0), the polar nature of the amide functionality may be more effective at interacting with the aqueous portion of the mobile phase and forming the stagnant water layer required for HILIC chromatography.
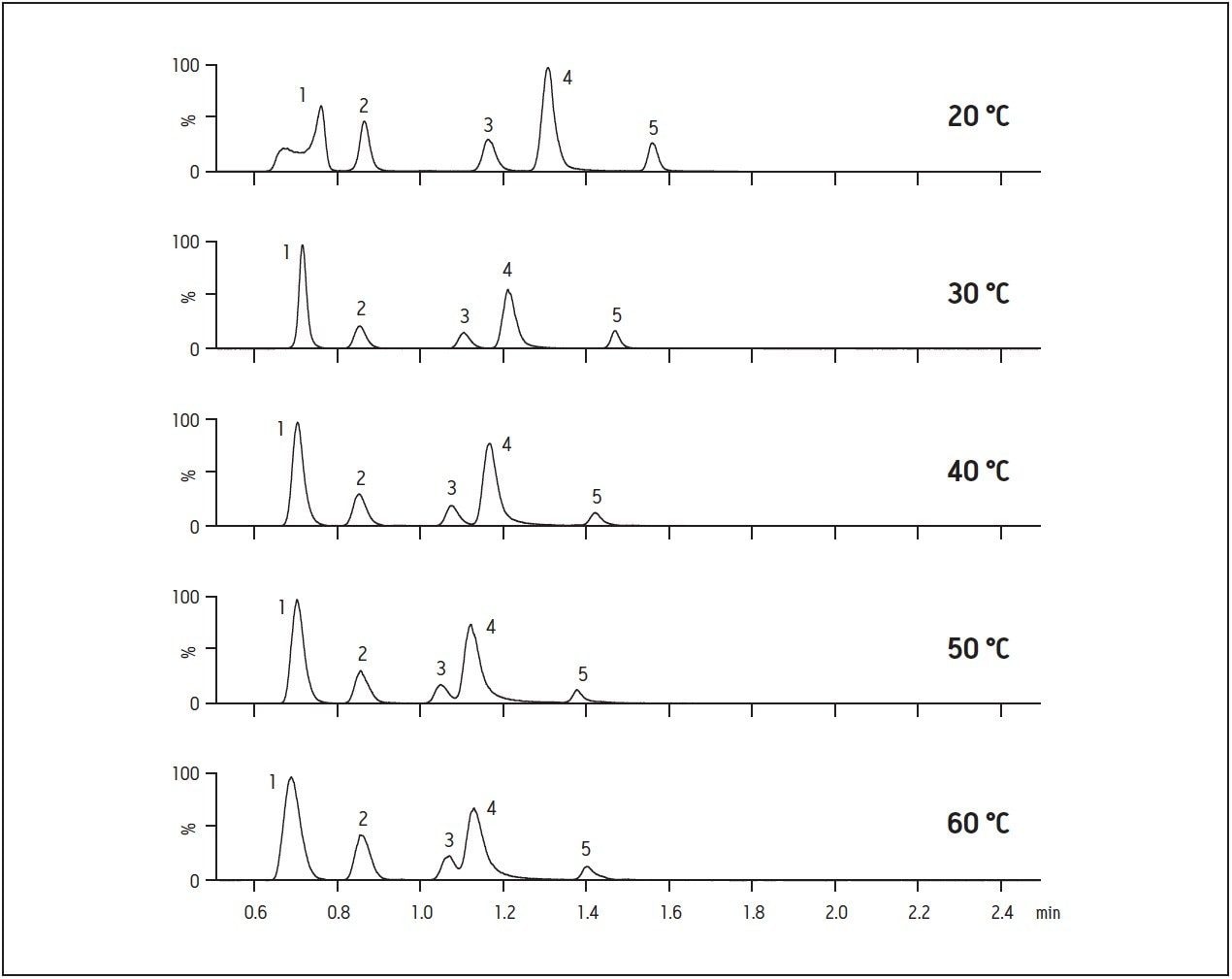
HILIC chromatography can be quite sensitive to temperature, and both decreases and increases in retention have been seen with temperature increases[12, 13]. Figure 2 shows chromatograms of monoamines run under the mobile phase conditions used in Figure 1A at different temperatures. In general, as temperature increased, the resolution between peaks decreased. At 40 °C, there is a decrease in the resolution between dopamine and epinephrine and at 60 °C, baseline separation has clearly been lost. Interestingly, when the column was cooled to 20 °C, there was a significant loss of peak shape for NMS. As this figure clearly shows, 30 °C provided the optimum balance of speed, resolution, and peak shape for all analytes.
The development of HILIC chromatography for the analysis of monoamine neurotransmitters using a 2.5 μm particle column is detailed. Optimizing the ionic strength of the organic mobile phase proved critical to maximizing chromatographic performance, but needed to be balanced with increases in aqueous content to ensure complete solubility. The superior performance of the XBridge BEH Amide XP column demonstrates the importance of stationary phase choice considerations during HILIC method development. Column temperature is also shown to be a crucial consideration for optimizing chromatography. These results show that retention, separation and resolution of even the most polar compounds (epinephrine and norepinephrine) can be readily achieved. The intermediate length of the column (75 mm) combined with the relatively low backpressures characteristic of HILIC analyses should afford ample flexibility to adapt this method as needed.
720004389, June 2012