This application note describes the use of the ACQUITY QDa module, a small but robust, simple-to-use mass detector, to quantitate non-chromophoric memantine HCl, a drug commonly used to treat dementia often associated with Alzheimer’s disease.
The analysis of compounds that lack UV chromophores or that have low UV-extinction coefficients can be challenging. Because these compounds cannot be directly detected by UV, their identification and measurement must depend on alternate methods. For all pharmaceutical products, it is particularly important to correctly identify its components, for failing to do so can compromise the drug’s safety, efficacy, or both.
For components that exhibit poor UV absorbance or none, samples often require pre-or post-column derivatization, for UV detection. In the case of memantine hydrochloride, a non-chromophoric drug compound, several methods are found in the literature for quantitative determination in dosage. Few methods for determining memantine HCl in drug formulations have been reported, including HPLC methods with UV-detection by means of a pre-column derivatization technique1,2 and GC-FID methods.3,4 While effective and sensitive, these methods are not ideal for routine testing in a quality control laboratory. They require tedious and complex pre-column derivatization procedures or additional instrumental analysis such as gas chromatography. The lack of straightforward methods can lead to additional undesirable variability in response. This often requires additional method-development time, robustness testing, user training, and monitoring in order to improve the quality of the assay. Mass detection, on the other hand, enables quick and accurate determination of non-chromophoric compounds, and can eliminate the need for complex sample-preparation procedures.
In this application note, we describe the use of the ACQUITY QDa module, a small but robust, simple-to-use mass detector, to quantitate non-chromophoric memantine HCl, a drug commonly used to treat dementia often associated with Alzheimer’s disease. Here, we present a UPLC method coupled with the ACQUITY QDa Detector to quantitatively determine memantine HCl in the drug tablet formulation. We will demonstrate the system suitability, method linearity, and specificity achievable with mass detection for routine assays.
Overall, mass detection provides quick identification and analysis of non-chromophoric compounds, accurate and reliable results. Thus, mass detection is complementary to UV techniques and suitable for routine testing in the QC laboratory.
|
LC system: |
ACQUITY UPLC H-Class |
|
Column: |
ACQUITY UPLC CORTECS C18+, 2.1 x 50 mm, 1.6 μm |
|
Column temp.: |
45 °C |
|
Flow rate: |
0.6 mL/min |
|
Injection volume: |
1.0 μL |
|
Solvent A: |
125 mM Formic acid in water |
|
Solvent B: |
Water |
|
Solvent C: |
Acetonitrile |
|
Separation: |
Gradient |
|
Step |
Time(min) |
Solvent A(%) |
Solvent B(%) |
Solvent C(%) |
Curve |
|---|---|---|---|---|---|
|
1 |
Initial |
10.0 |
85.0 |
5.0 |
Initial |
|
2 |
2.5 |
10.0 |
42.5 |
47.5 |
6 |
|
3 |
2.6 |
10.0 |
0.0 |
90.0 |
6 |
|
4 |
3.1 |
10.0 |
0.0 |
90.0 |
6 |
|
5 |
3.2 |
10.0 |
85.0 |
5.0 |
6 |
|
6 |
5.0 |
10.0 |
85.0 |
5.0 |
6 |
|
Purge/Sample wash: |
50:50 water/methanol |
|
Seal wash: |
90:10 water/acetonitrile |
|
UV detector: |
ACQUITY UPLC PDA: 210–400 nm (derived at 210 nm) |
|
Mass detector: |
ACQUITY QDa (Extended Performance) |
|
Ionization mode: |
ESI+ |
|
MS Acquisition range: |
100 – 300 Da |
|
Single Ion Recording: |
180.2 Da |
|
Sampling rate: |
10 pts/s |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
15 V |
|
Probe temperature: |
600 °C |
|
Data: |
Centroid |
|
System Control, Data Acquisition, and Analysis: |
Empower 3 FR2 CDS Software |
The memantine HCl stock solution was prepared in methanol at a concentration of 1.0 mg/mL. The stock solution was then diluted with standard diluent (10:90 methanol/water) to a working concentration of 0.005 mg/mL. The working standard solution was used to prepared linearity standards by dilution with standard diluent (10:90 methanol/water). Linearity standards were prepared at these concentrations: 0.05, 0.10, 0.15, 0.25, 0.50, 0.75, and 1.00 μg/mL.
Stock sample solutions were prepared by dissolving tablets containing 10 mg of memantine HCl in 50:50 0.1 N HCl/ethanol,* to a concentration of 1.0 mg/mL. The solutions were sonicated and centrifuged, at 3500 rpm, for 30 minutes. Finally, the solutions were filtered through 0.2-μm GHP membrane syringe filters* and diluted with sample diluent (0.1N HCl) to the working concentration of 0.75 μg/mL.
*Final method, other dissolution and filtering conditions tested and presented in this application note.
Memantine HCl is a tricyclic amine that lacks the chromophores required for UV detection (Figure 1). Thus it cannot be directly detected by UV. Nevertheless, it is readily ionizable, producing a robust MS signal on the ACQUITY QDa Detector. Figure 2 shows several detector traces. As expected, the UV trace at 210 nm for memantine at 1 μg/mL shows no discernible peak. (Figure 2a.)
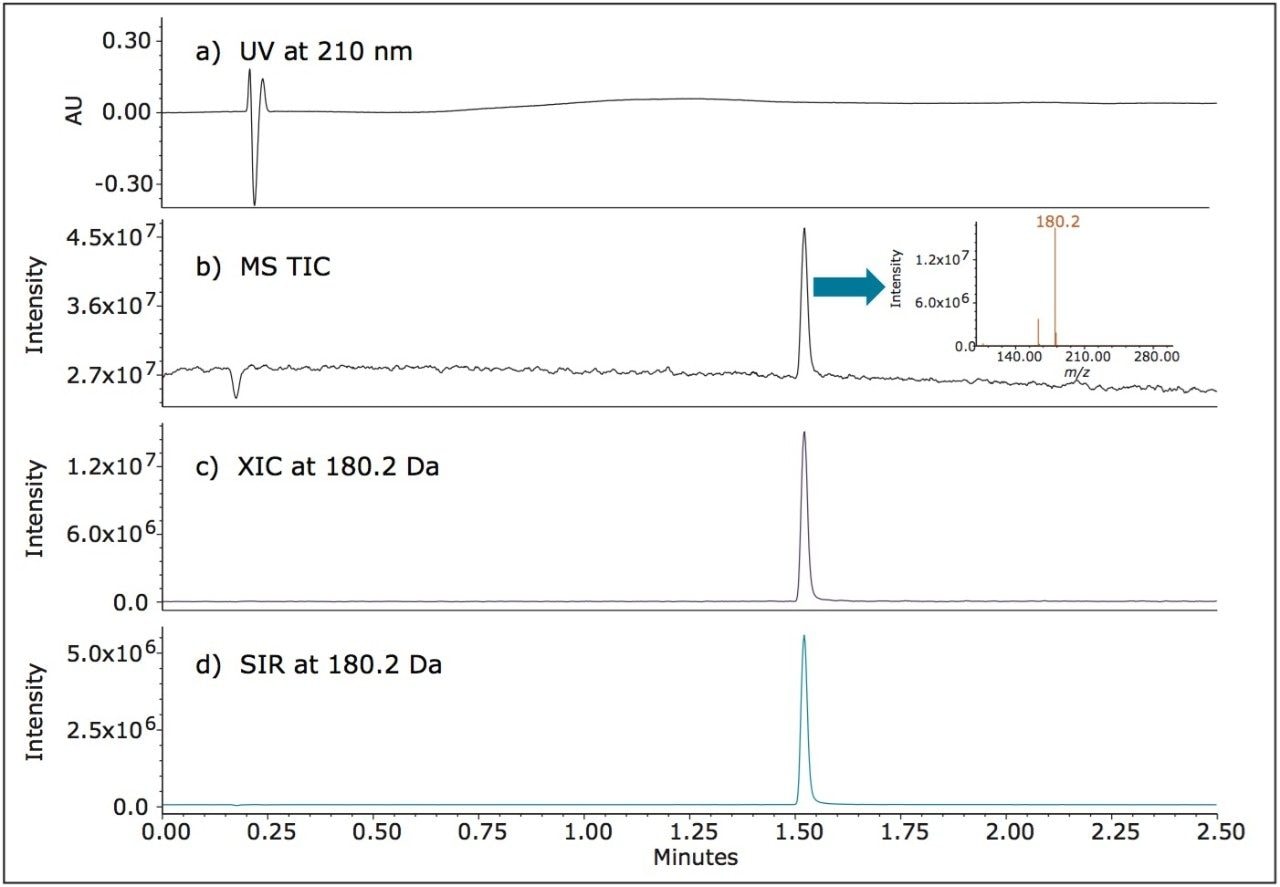
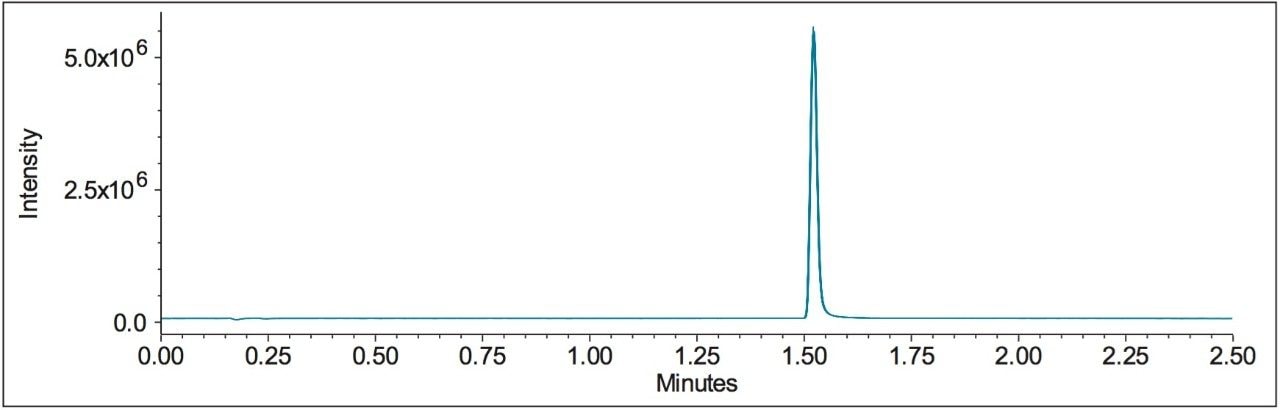
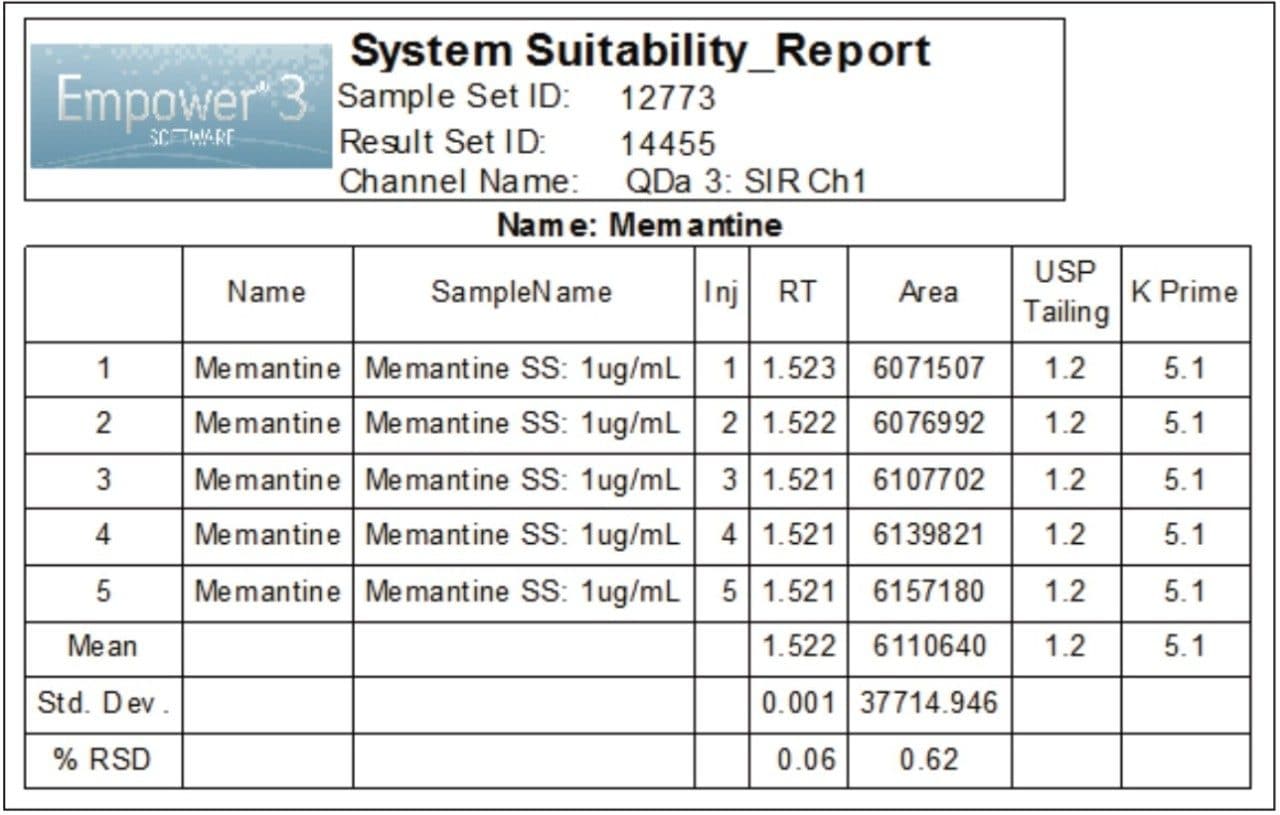
Performance of the UPLC method was verified by evaluating repeatability of five replicate injections of the 1 μg/mL standard (Figure 3) made according to the specifications defined in the USP General Chapter, <621> “Chromatography”.5 The UPLC system suitability results, processed using SIR mass data at 180.2 Da, are shown in Figure 4. The retention times and area repeatability were well within the USP specification of less than 2% RSD.
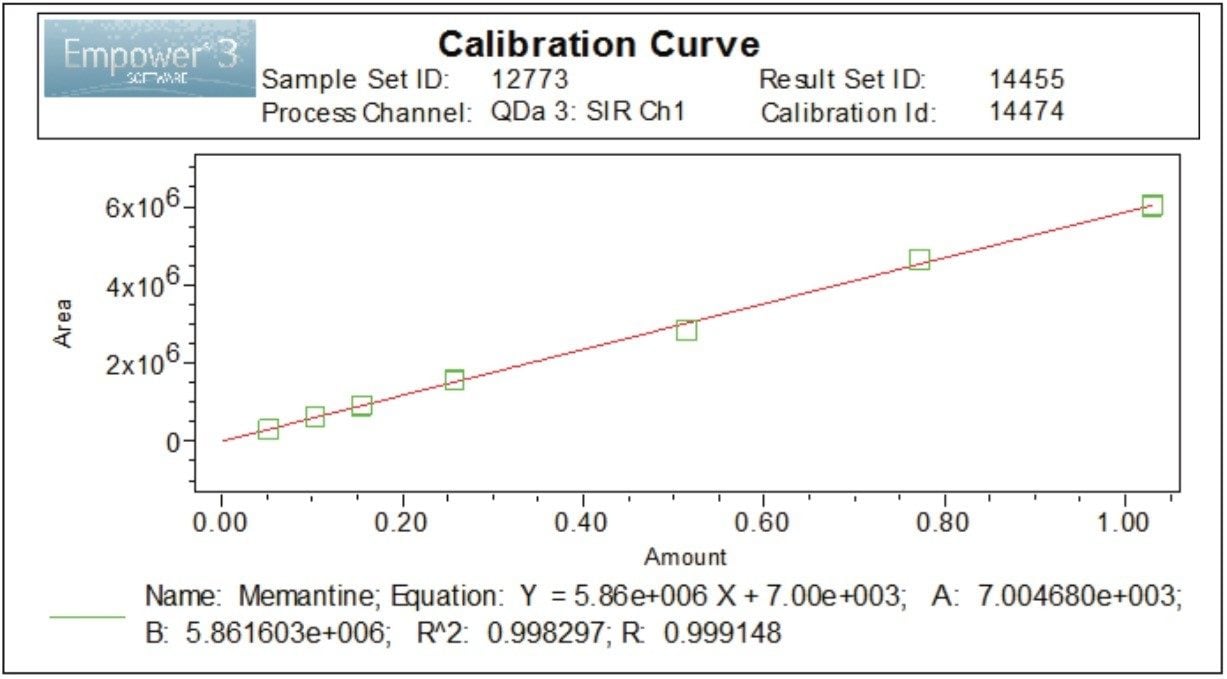
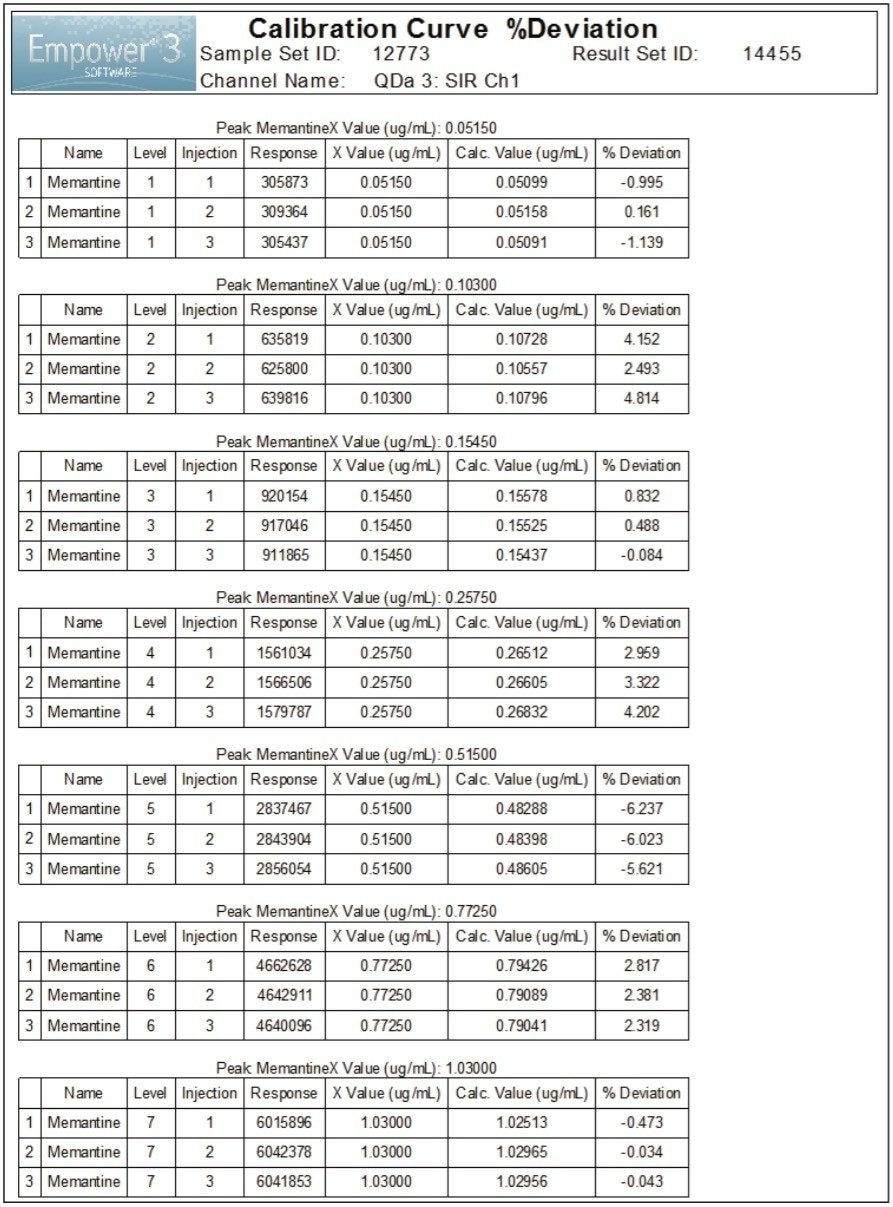
Linearity of the method for memantine HCl with mass detection was evaluated over seven concentrations ranging from 0.05 μg/mL to 1.0 μg/mL. The method showed good linear correlation between the peak areas and concentrations of memantine HCl, with correlation coefficients (R2) ≥0.998 (Figure 5). In addition, the percent deviation of the calculated x values or concentrations were less than 7.0% (Figure 6).
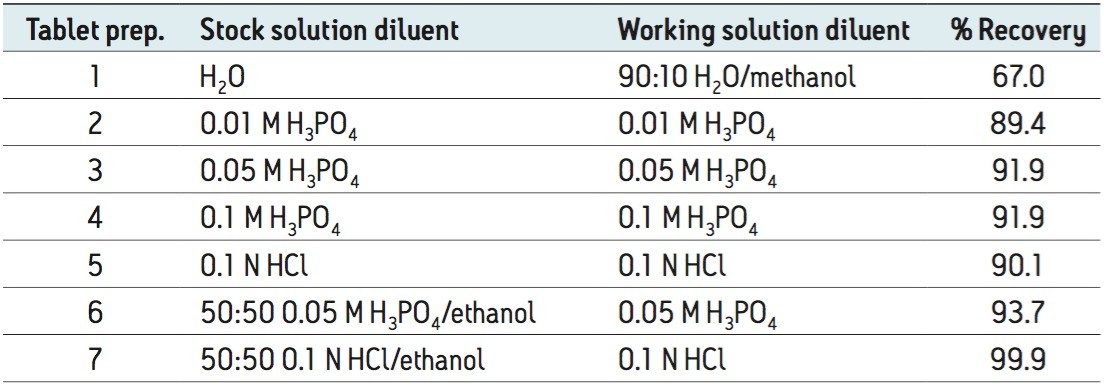
The UPLC-MS method was then used to analyze commercially available memantine HCl tablets, to demonstrate specificity for routine assay. To develop a sample preparation procedure for the tablets, various sample diluents and filters were considered, with a goal of meeting the acceptance criteria for recovery, defined in the USP Monograph for Memantine Hydrochloride Tablets.4 According to that monograph, “Memantine HCl Tablets contain an amount of memantine hydrochloride equivalent to NLT 90.0% and NMT 110.0% of the labeled amount of memantine hydrochloride (C12H21N•HCl).”
Sample diluents investigated during the study are shown in Table 1. Separate sample solutions were prepared by dissolving tablets using all diluents and then sonicating and centrifuging the stock solutions. They were then filtered through 0.2 μm GHP syringe filters before undergoing dilution to the working concentration. The data show that preparation in diluent containing 50:50 0.1 N HCl/ethanol resulted in the highest recovery of memantine HCl: 99.9%.
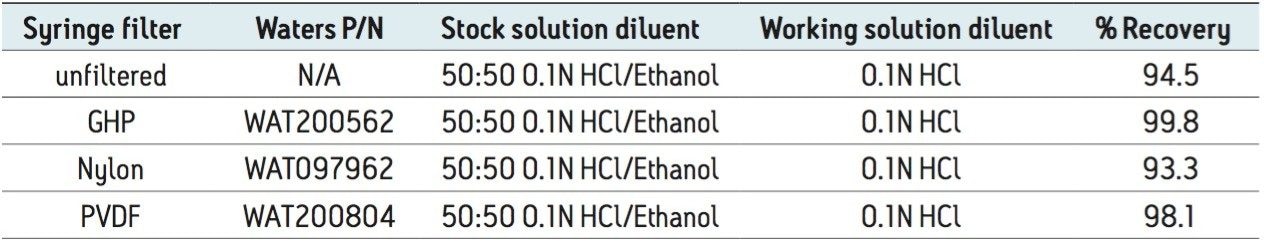
In addition to diluent, the effect on recovery of filter type was also evaluated. Particulates or insoluble materials present in the sample solution can interfere with chromatography or effect a poor recovery. Removal of particulates through sample filtration can often improve the recovery. The effect on recovery of three different filter membranes, as compared with no filtration (control), was studied (Table 2). Stock sample solutions prepared in 50:50 0.1 N HCl/ethanol were filtered through three, 0.2-μm, syringe filters. As shown in Table 2, filtration through the GHP-membrane syringe recovered the memantine HCl from the tablet formulation most effectively. Significantly, recovery of the compound from the unfiltered sample solution was lower than it was from the solution filtered through the GHP filter. These results show that sample filtration and prudent selection of filter type are necessary to maximize recovery.
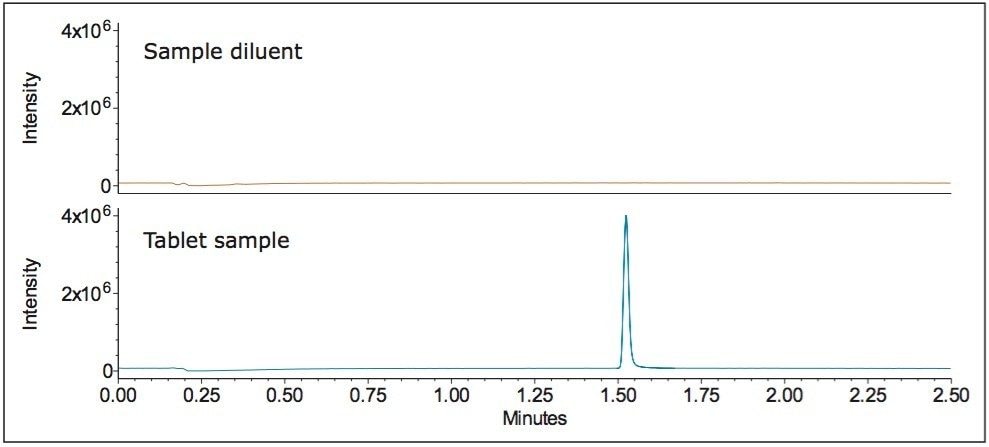
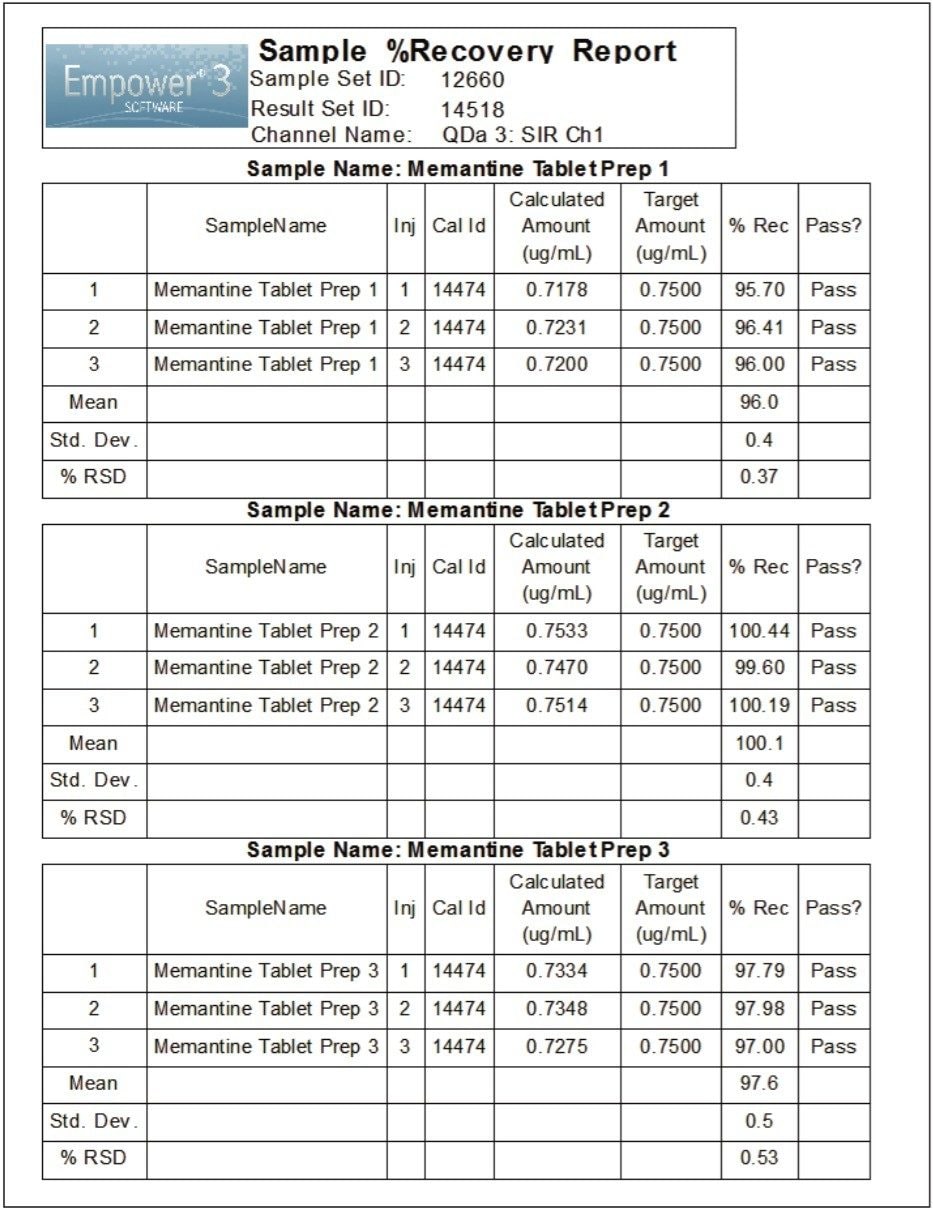
For sample analysis, three separate preparations of tablets were tested for the assay of memantine HCl. An example of the chromatographic data of sample diluent and tablet sample solution acquired using the ACQUITY QDa Detector with SIR at 180.2 Da is displayed in Figure 7. The average percent recovery of memantine HCl for the three preparations ranged from 96.0 to 100.1% (Figure 8), which meets the USP acceptance criteria of 90.0–100.0%, defined in the USP Monograph for memantine HCl tablets.
Mass detection using the ACQUITY QDa Detector enabled detection and quantitative determination of non-chromophoric memantine HCl. System suitability and method linearity calculated using mass data were excellent. For analysis of tablets, a non-derivatization sample preparation procedure for quantitative determination of memantine HCl was developed, eliminating the need for a complex and tedious pre-column derivatization protocol before the analysis. The use of SIR allowed the selection of a target mass, reducing interferences in the analysis of formulated samples.
Overall, the ACQUITY QDa Mass Detector is a robust, simple to use, orthogonal, detection technique to UV detection. It provides accurate and reliable results, making this technology ideal for routine testing in the QC laboratory.
720005179, September 2014