This application note describes an LC-MRM method developed on the ionKey/MS System that is able to detect very low levels of OT in human plasma, at an LLOQ of 10 pg/mL.
Quantitative LC-MRM (multiple reaction monitoring) methods for small molecule drugs are used to provide bioanalytical support in various stages of drug discovery and development. These methods can routinely achieve lower limits of quantification (LLOQ) in the range of 50 to 100 pg/mL, in various biological matrices, using analytical-scale chromatography (e.g., 2.1 mm I.D. UPLC Columns). In the case of peptide therapeutics, these assays are more challenging because lower LLOQs are often required.
One method that allows significant sensitivity enhancements is to operate the LC-MS system at lower LC flow rates, which provides reduced chromatographic peak volumes and increased ionization efficiency with electrospray ionization mass spectrometry (ESI-MS). However, most “homemade” capillary-flow LC-MS configurations suffer from a lack of robustness and are often not able to provide adequate sample throughput. The ionKey/MS System is an integrated capillary-flow microfluidic system that is designed to operate in the flow range of 1 to 5 µL/min, which can provide a 10- to 20-fold increase in sensitivity for therapeutic peptides when compared to conventional analytical-scale LC-MS platforms.
Oxytocin (OT) is a mammalian, 9-amino-acid cyclic peptide (CYIQNCPLG-NH2) that acts primarily as a neurotransmitter in the brain. Quantitative measurement of endogenous OT in biological samples is very challenging, because it is present at low pg/ml concentrations in human plasma.1 ELISA2-3 and mass spectrometry assays3-6 have been previously reported for measurement of endogenous OT levels. However, the LLOQ of commercial ELISA assays for OT is above the endogenous level. Several methods using mass spectrometry have been developed recently using affinity capture for OT enrichment,3 two-dimensional (2D) LC-MS/MS using a tandem quadrupole MS in conjunction with large-volume sample extraction (1.4 mL human plasma),4 or 2D-LC-MS/MS with large volume injection5 to achieve the required sensitivity.
Here we report an LC-MRM method developed on the ionKey/MS System that is able to detect very low levels of OT in human plasma, at an LLOQ of 10 pg/mL.
|
LC system: |
ACQUITY UPLC M-Class System |
|
Separation device: |
iKey BEH C18 Separation Device, 130Å, 1.7 μm, 150 μm x 100 mm (p/n 186007258) |
|
Mobile phase A: |
0.1% Formic acid (FA) in water |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile (ACN) |
|
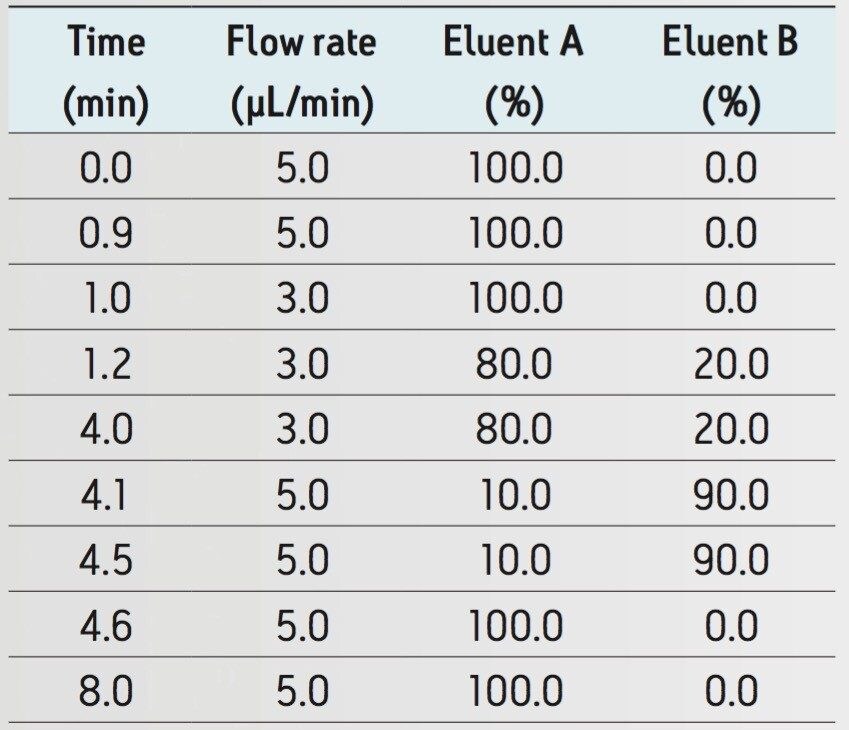
Flow rate and gradient: |
See Table 1 |
|
iKey temp.: |
60 °C |
|
Sample temp.: |
10 °C |
|
Injection vol.: |
3 μL |
|
Total run time: |
8 min |
|
MS system: |
Xevo TQ-S with ionKey Source |
|
Ionization mode: |
ESI+ |
|
ESI voltage: |
3.2 kV |
|
Source temp.: |
100 °C |
|
Nebulizing gas pressure: |
0.2 bar |
|
MRM transitions: |
See Table 2 |
|
Cone voltage: |
See Table 2 |
|
Collision energy: |
See Table 2 |
|
Chromatography software: |
MassLynx Software |
|
Quantification software: |
Target Lynx Application Manager |
Oxytocin (Sigma Aldrich, St. Louis, MO, USA) was spiked in 200 µL of K2-EDTA human plasma (Bioreclamation, East Meadow, NY, USA) at the following concentrations: 10, 20, 100, 200, 1,000, 10,000, and 20,000 pg/mL. 13C15N-isotopically labeled OT (CYIQNCPLG-NH2, Sigma Aldrich) was added as an internal standard (IS) at 100 pg/mL in all samples. Protein precipitation was performed after adding 200 µL of acetonitrile to achieve 1:1 sample dilution. Samples were vortexed briefly (5–10 sec), and then were spun at 4,000 RPM for 15 minutes (at room temperature) using a 5810R centrifuge (Eppendorf, Hauppauge, NY, USA). The supernatant (200 µL) was diluted with 1.8 mL of 4% H3PO4 and sample clean-up was performed using an Oasis HLB 96-well Plate, 5 mg sorbent per well, 30 µm Particle Size (p/n 186000309). The HLB extraction protocol is provided in Figure 1. After 1:1 dilution with 0.1% formic acid in DI water, 3 µL of sample were injected on the ionKey/MS System.

One of the first experiments performed when developing an LC-MRM assay for a peptide therapeutic is to record a full scan ESI-MS spectrum of the analyte to establish its most abundant precursor. Figure 2A shows the ESI-MS spectrum of OT (average of 10 scans) recorded during analyte elution at a flow rate of 3 µL/min, after the injection of a standard containing 10 ng/mL OT, on the ionKey/MS System. Surprisingly, the most intense peptide precursor is the singly charged species at m/z = 1007.4 and not the expected doubly protonated ion at m/z = 504.2. This observation can be explained by the fact that OT contains a disulfide bond that restricts peptide protonation. In a separate LC injection, the MS/MS spectrum of OT (10 average scans) displayed in Figure 2B was produced from the same OT standard. Fragmentation of the singly charged precursor using a collision energy of 25 V produced a very abundant fragment ion at m/z = 723.3 assigned to a b6 ion. The best responding MRM transition (1007.4 ⇒ 723.3) was then optimized in terms of cone voltage and collision energy. The optimized parameters for OT and its internal standard are summarized in Table 2.
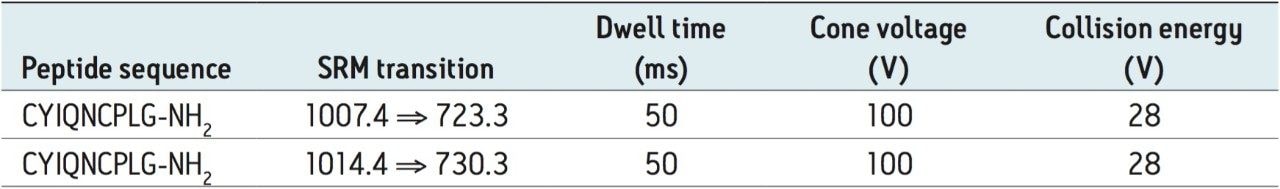
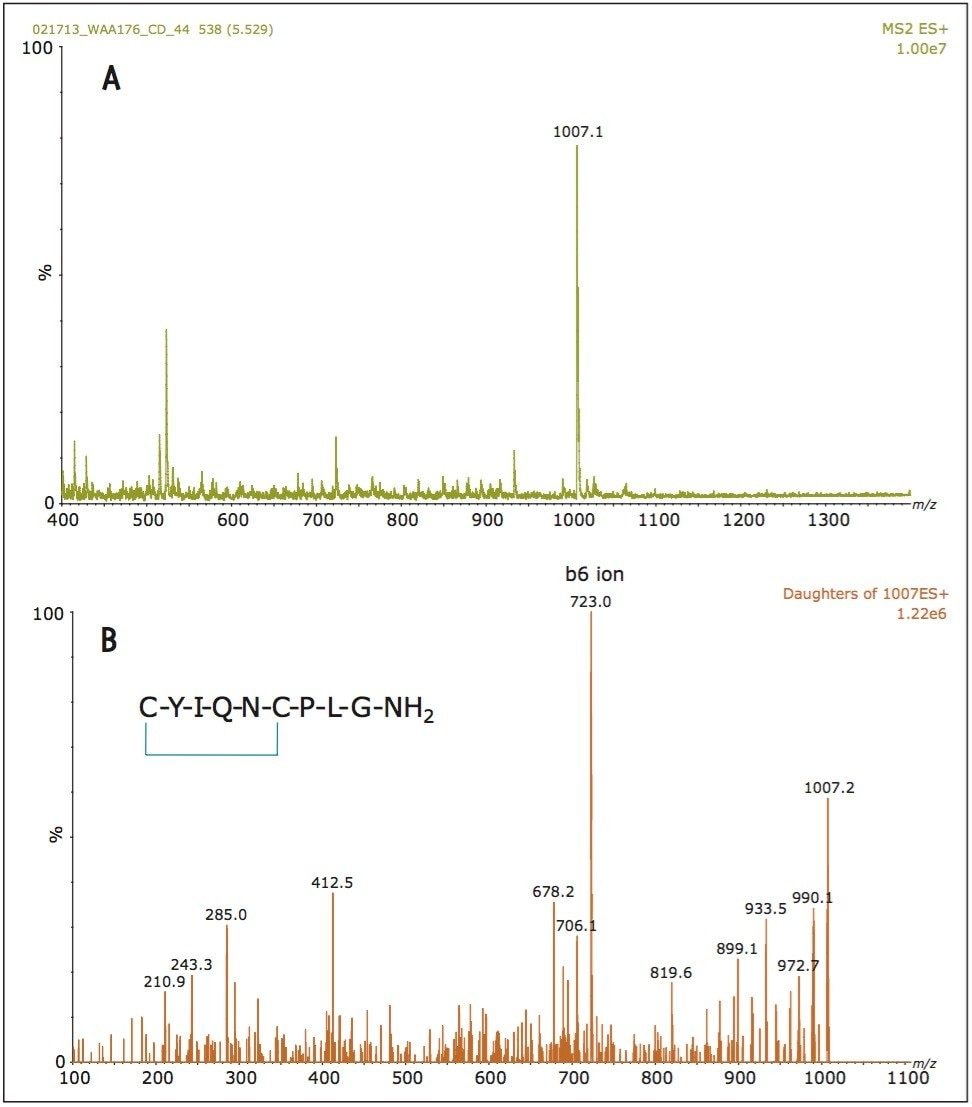
Figure 2A. ESI-MS spectrum of oxytocin (OT).
Figure 2B. ESI-MS/MS spectrum of OT produced by the fragmentation of the singly charged precursor using a CE of 28 V.
The sample preparation protocol was optimized to achieve efficient removal of sample matrix. While SPE is typically used as a one-step sample clean-up in many peptide therapeutic protocols, the amount of residual sample matrix can still be significant and can cause poor chromatography and decreased assay robustness after hundreds of sample injections on capillary-scale chromatography.
Protein precipitation offers an efficient way to decrease the amount of protein component matrix from plasma and it was used here in conjunction with SPE to increase method robustness. In addition, protein precipitation also provides a good opportunity to check for protein binding, as many peptide therapeutics are typical substrates for plasma proteins.
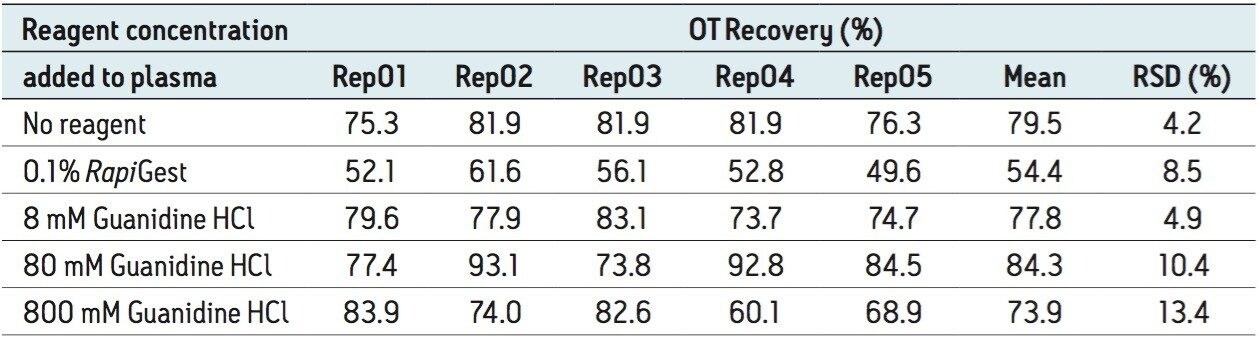
Protein binding can have significant negative effects on the ability of the LC-MRM assay to quantify the therapeutic peptide. Protein binding can be disrupted by surfactants (e.g., RapiGest) or protein denaturants (e.g., guanidine hydrochloride). In the case of oxytocin, protein binding was evaluated by comparing analyte recovery after protein precipitation in the presence and absence of several protein-binding disrupting reagents. Peak areas of OT, obtained for pre-spiked and post-spiked protein precipitated plasma samples, were used to calculate the OT recovery and the results are summarized in Table 3. With the exception of RapiGest recoveries, the values presented in this table indicate high analyte recoveries (70% to 85%) regardless of the precipitation protocol. Clearly, OT is not affected by protein binding and protein precipitation can be safely performed in the absence of detergents or protein denaturants.
OT was spiked in 200 µL of K2-EDTA human plasma at the following concentrations: 10, 20, 100, 200, 1,000, 10,000, and 20,000 pg/mL. 13C15N-isotopically labeled OT (CYIQNCPLG-NH2) was added as an IS at 100 pg/mL in all samples. Following protein precipitation, the supernatant was diluted 10-fold with 4% H3PO4 and SPE was performed on an Oasis HLB Sorbent to isolate the analyte and the IS. Extracts were diluted 1:1 with 0.1% FA and injected on the ionKey/MS System.
The LLOQ of the OT assay was 10 pg/mL and the MRM chromatograms recorded at the LLOQ level are displayed in Figure 3. The chromatograms shown in Figure 3A represent three successive injections of solvent A blank (0.1% FA), human plasma blank and 10 pg/mL OT spiked in human plasma. The analyte signal detected in the plasma blank was probably produced by the endogenous oxytocin present in human plasma. The OT peak area in the spiked sample is approximately twice the area of the blank signal. Replicate injections at the LLOQ level (Figure 3B) indicate good data reproducibility, with a peak area RSD of 13.2%.

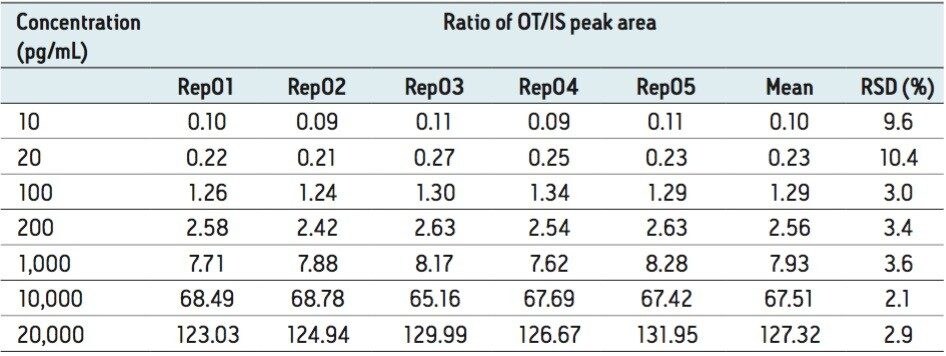
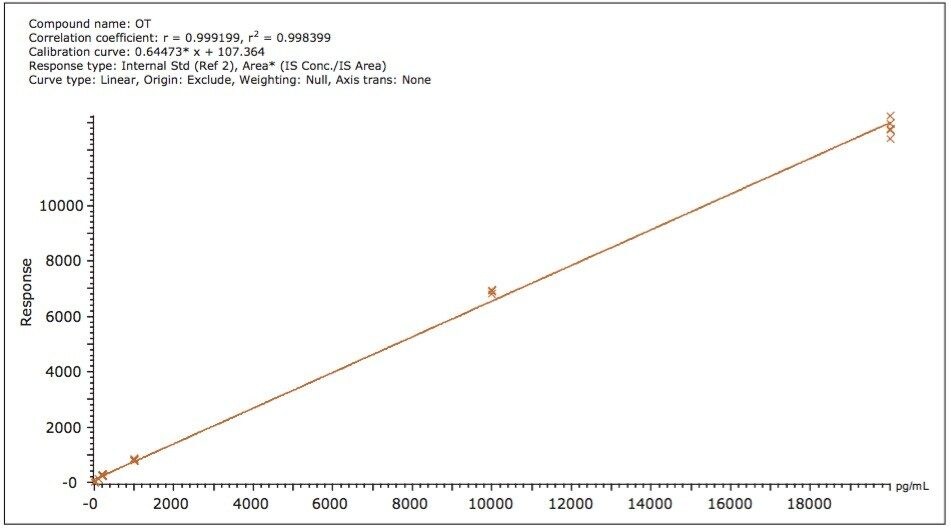
The assay was tested for dynamic range exceeding three orders of magnitude (10 to 20,000 pg/mL oxytocin in human plasma) and a table containing the peak area ratios (OT area/IS area) along with the corresponding RSDs is presented in Table 4. The TargetLynx calibration curve for the same concentration range is shown in Figure 4 and has very good linearity (r2=0.998).
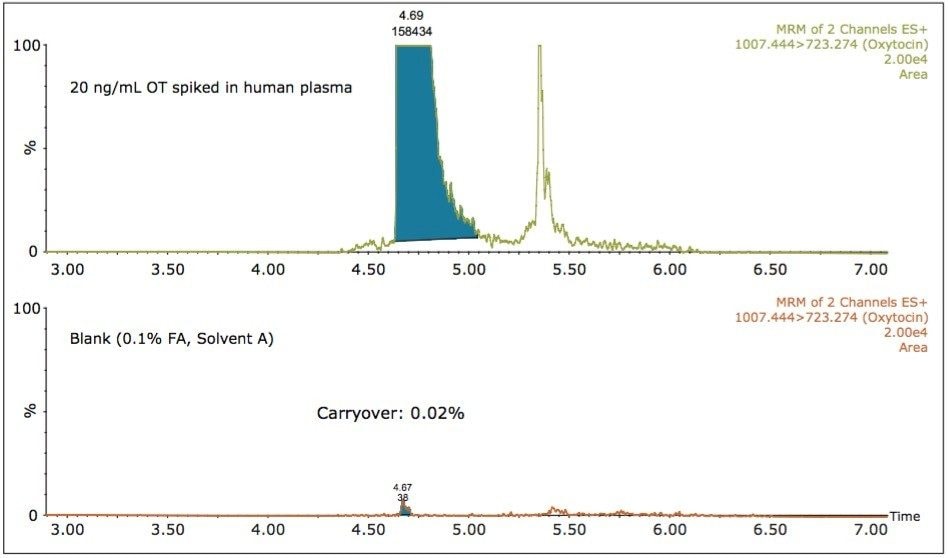
The carryover of the assay was evaluated by injecting a blank (0.1% FA, solvent A) following the injection of the highest concentration spiked sample (20 ng/mL OT spiked in human plasma). According to the data displayed in Figure 5, the analyte carryover was 0.02% and the peak area recorded for the blank sample was approximately two-fold below the peak area at the LLOQ level.
The quantification method developed with the ionKey/MS System is simple, specific, robust and has been implemented in a high-throughput (96-well plate) format. In addition, the ionKey/MS System offers significant advantages in terms of operating costs when compared to analytical scale LC-MRM: the cost of mobile phase solvents are typically reduced by 100-fold and sample preparation costs are typically reduced 5- to 10-fold because smaller injection volumes are required (1 to 5 µL).
720005011, February 2016