This application demonstrates two approaches for making allowed changes to an isocratic USP method involving modern column particles such as that found in solid-core CORTECS Columns.
Using the “equivalent L/dp” guideline is an easy way to modernize a USP method and greatly improve the speed of analysis. With the “equivalent N” guideline, the analyst can realize still further analysis speed improvements from even shorter, highly efficient columns in the CORTECS family.
Many United States Pharmacopeia (USP) monograph LC methods were created years ago when longer columns, packed with larger fully-porous particle sorbents, were the norm. Methods using these columns can now be considered “outdated” in resolving power and speed. Switching the stationary-phase particles from larger to smaller and from fully-porous to solid-core can greatly improve method resolution and speed. Better resolution arises from the narrower peaks (higher efficiency) that these particles provide. Quicker methods originate in the ability to use shorter columns with such particles without sacrificing efficiency.
This application note illustrates how an analyst can use solid-core CORTECS Columns to modernize a USP method. We selected the dofetilide1 USP assay method for improvement and demonstrate two different allowed USP isocratic LC method changes to achieve much higher analysis speed and lower solvent consumption.
To change the stationary-phase particles used in a USP method, the analyst must consult USP General Chapter <621>. This section of the USP specifies the alterations to an LC method that are permissible without revalidation. For isocratic methods, the analyst can change the stationary-phase particle in one of two allowed ways.2 The first approach maintains an equivalent ratio of the column length, L, to the particle size (diameter), dp, in the range of -25% to +50% of the L/dp ratio3 specified in the USP method. The second way employs other combinations of L and dp that provide an equivalent number of theoretical plates (plate count, also called the column efficiency), N, within -25% to +50% of that measured for the original column specified in the method.
The “equivalent L/dp” guideline is based on eq 1 where, for isocratic LC methods, the column plate count can be estimated4 from the column length, particle size, and reduced plate height, h.
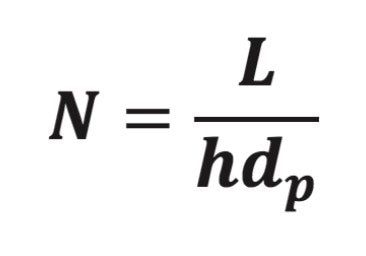
For chromatography of small molecules on well packed columns with fully-porous particles, h is approximately6 equal to 2. With h ≈ constant, it is justified to scale a USP method by L/dp to get an equivalent plate count, N, when the original stationary-phase particles and the replacement stationary-phase particles are both fully porous.
However, when solid-core particles replace fully-porous particles, h can decrease (in the range of 1.4 to 1.6), which increases the efficiency.7 In such cases, the “equivalent N” guideline may replace “equivalent L/dp” in modernizing USP methods. This requires actual plate count measurements on the USP method comparing the original and replacement columns packed with fully-porous and solid-core particles, respectively. The equation3 to measure USP plate count, N, is:
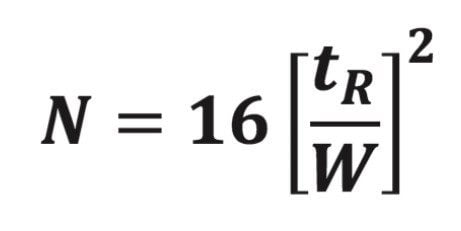
where tR is the analyte peak retention time and W is the analyte peak width at its base. The value of N, for each analyte in a USP method, can be easily measured using the System Suitability module of Waters Empower Chromatography Data Software.
During chromatography, the pressure drop across a column, ΔP, is given8 by eq 3, where F is the mobile phase flow rate, η is the mobile phase viscosity, r is the column radius, and εe is the column external porosity.
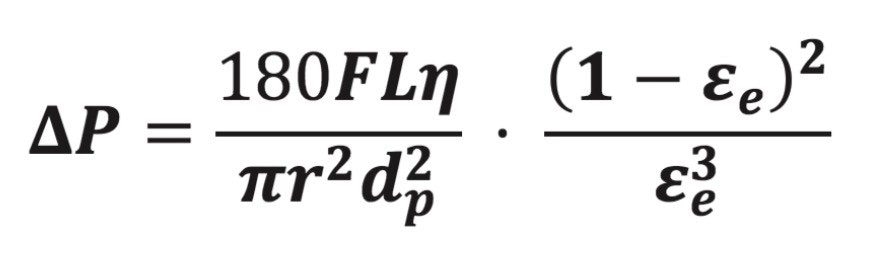
During USP method modernizations using “equivalent L/dp”, the pressure decrease at a given flow rate due to the shorter column length is small compared to the pressure increase from the smaller particles. The gain in analysis speed by using the shorter column with proportionally smaller dp therefore has a cost, in the form of higher column pressure. The magnitude of the pressure increase can be mitigated, however, if one changes the column external porosity. External porosity is a measure of how much the packed particles in a column resist the flow of liquid. It is a property that does not depend on the other parameters in eq 3. Solid-core particle columns have a higher external porosity (e.g. εe ≈ 0.39 for CORTECS) whereas fully porous particle columns have a lower external porosity (e.g. εe ≈ 0.37 for BEH C18). Holding constant all parameters of eq 3, except for external porosity, allows a plot of εe vs. ΔP. Figure 1 shows one such plot.
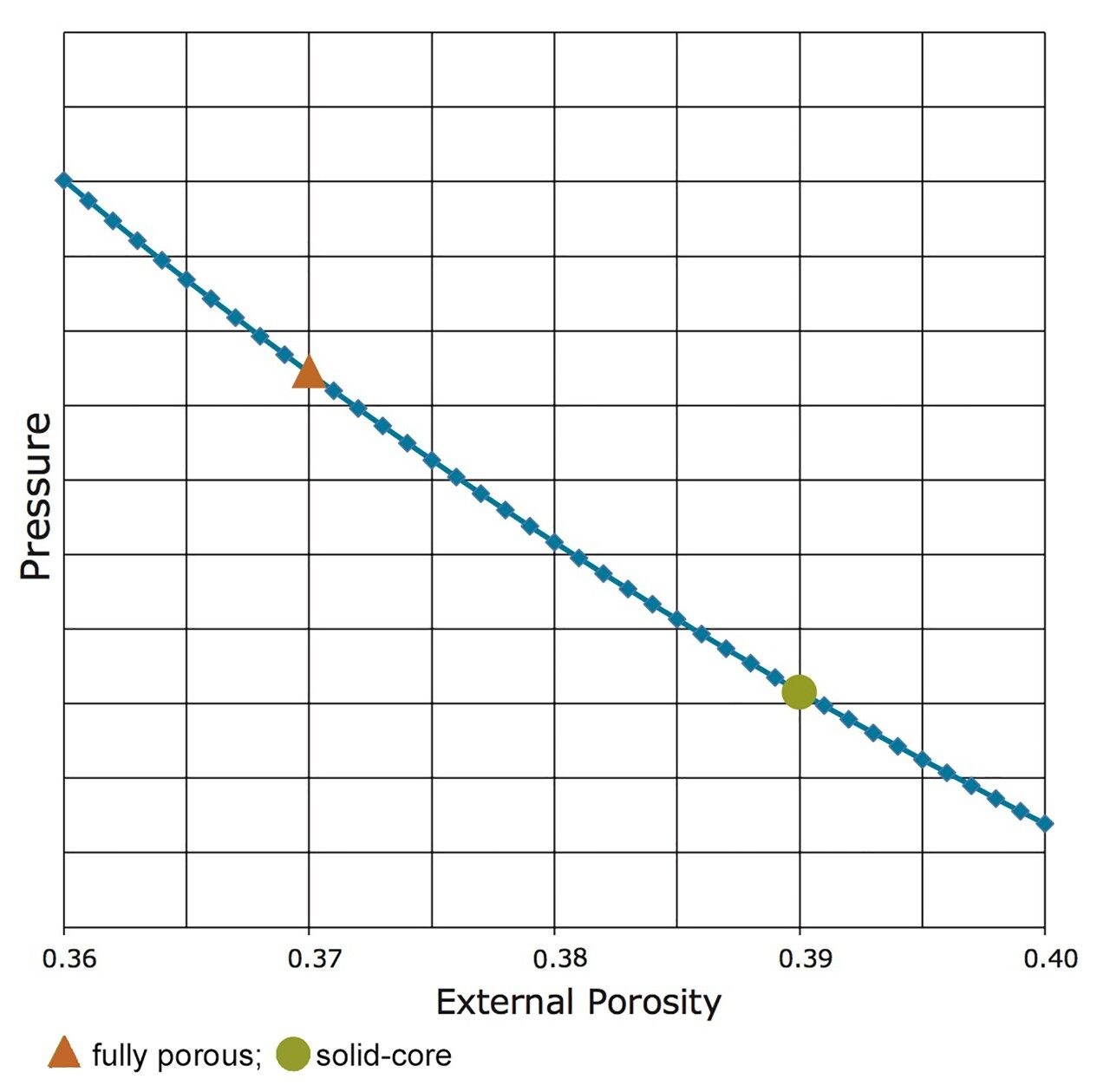
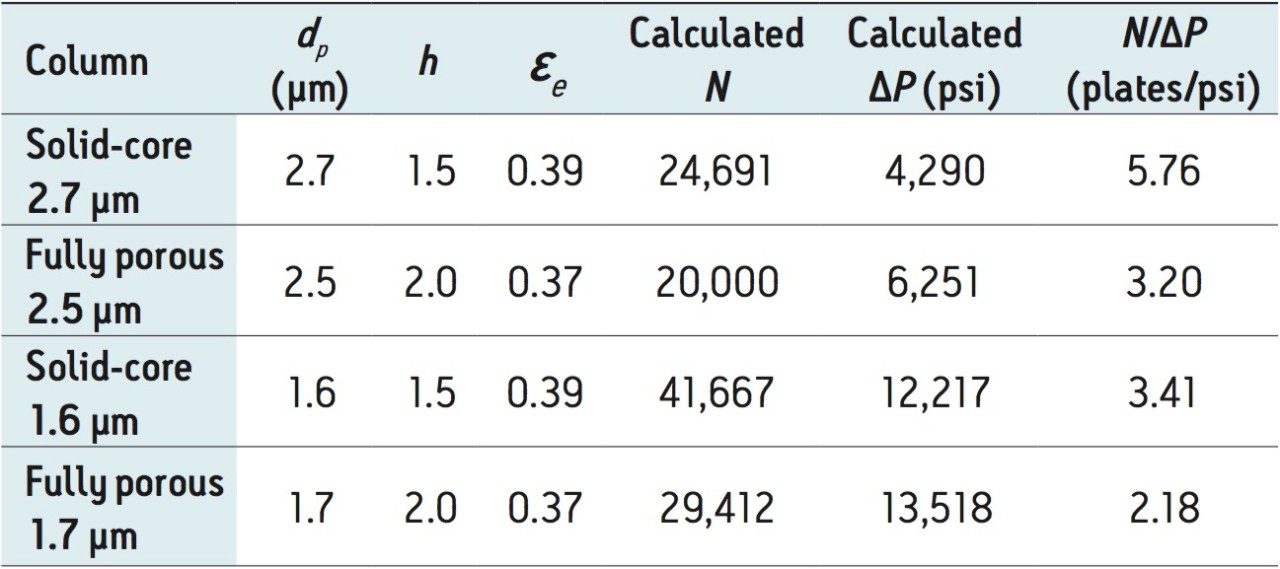
One can calculate the efficiency vs. pressure (N/ΔP) relationship for optimally packed fully-porous and solid-core columns from eqs 1 and 3. Table 1 gives some examples of this calculation with different column particles. The pattern is clear; a solid-core particle will always give more efficiency benefit for a given pressure cost.
A sample containing dofetilide (25 μg/mL) and dofetilide related compound A (0.5 μg/mL) was prepared with mobile phase as the diluent.
|
LC systems: |
Alliance HPLC and ACQUITY UPLC H-Class |
|
Data management: |
Empower 3 CDS |
|
Original compendial method conditions |
|
|
Column: |
Nova-Pak C8 Column, 60Å, 4 μm, 3.9 x 150 mm (p/n WAT035876) |
|
Mobile phase: |
Acetonitrile:buffer solution (1:3) |
|
Buffer solution: |
1.36 g monobasic potassium phosphate and 5 mg ascorbic acid in 1 L water, adjusted with 0.01 M potassium hydroxide solution to pH 7.0 |
|
Separation technique: |
Isocratic |
|
Flow rate: |
1.00 mL/min |
|
Column temp.: |
30 °C |
|
Detection (UV): |
230 nm |
|
Injection volume: |
50 μL |
|
(only changes are listed) |
|
|
Columns: |
CORTECS C8 Column, 90Å, 2.7 μm, 3.0 x 100 mm (p/n 186008361) CORTECS C8 Column, 90Å, 2.7 μm, 3.0 x 75 mm (p/n 186008360) CORTECS C8 Column, 90Å, 2.7 μm, 3.0 x 50 mm (p/n 186008359) CORTECS UPLC C8 Column, 90Å, 1.6 μm, 3.0 x 50 mm (p/n 186008409) CORTECS UPLC C8 Column, 90Å, 1.6 μm, 3.0 x 30 mm (p/n 186008408) |
|
Flow rate: |
0.88 mL/min (2.7 μm columns) 1.30 mL/min (1.6 μm columns) |
|
Injection volume:5 |
19.8 μL (3.0 x 100 mm columns) 14.8 μL (3.0 x 75 mm columns) 9.9 μL (3.0 x 50 mm columns) 5.9 μL (3.0 x 30 mm columns) |
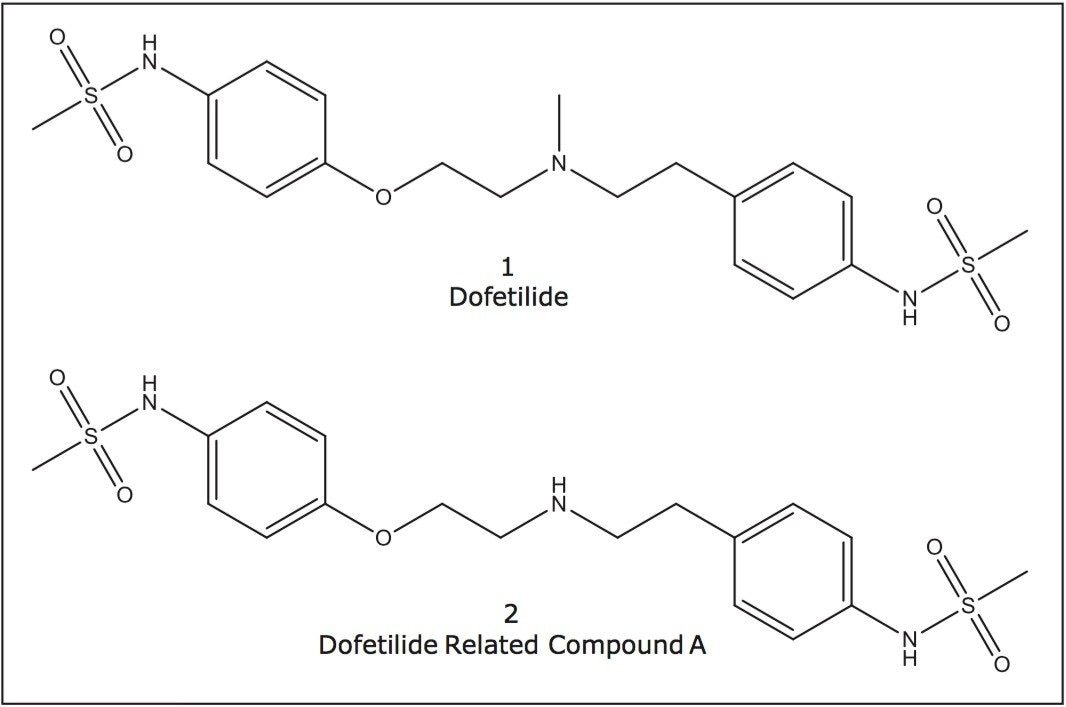
Dofetilide is a prescription pharmaceutical given to treat patients with irregular heartbeats. It is a class III antiarrhythmic agent that specifically blocks rapid potassium channels9 and is manufactured by Pfizer under the brand name TIKOSYN. The USP assay method uses both dofetilide, 1, and its related desmethyl compound, 2, for analysis, Figure 2.
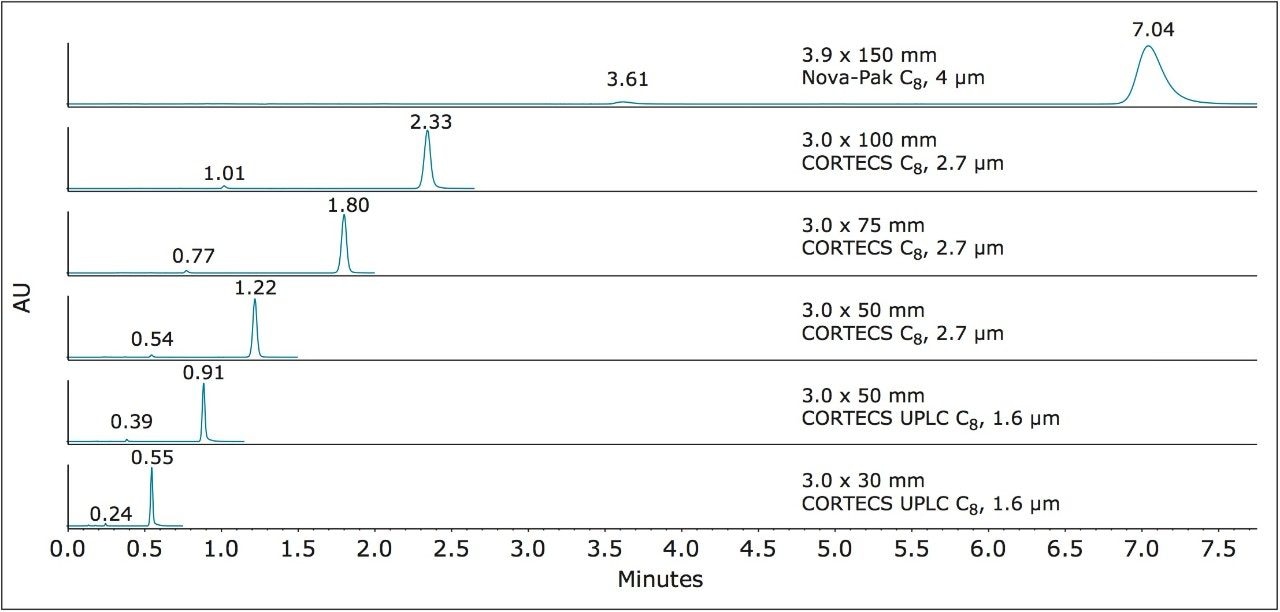
This method requires an L7 (C8) column. In particular, a Waters Nova-Pak C8 Column, 4 μm,3.9 x 150 mm (p/n WAT035876) with an L/dp of 37,500 was originally used. Since this original USP method column is designed for HPLC analysis, it was run on the Alliance HPLC System. The chromatogram and results, Figure 3 and Table 2, will be used as the compendial reference for the analytical method modernization.
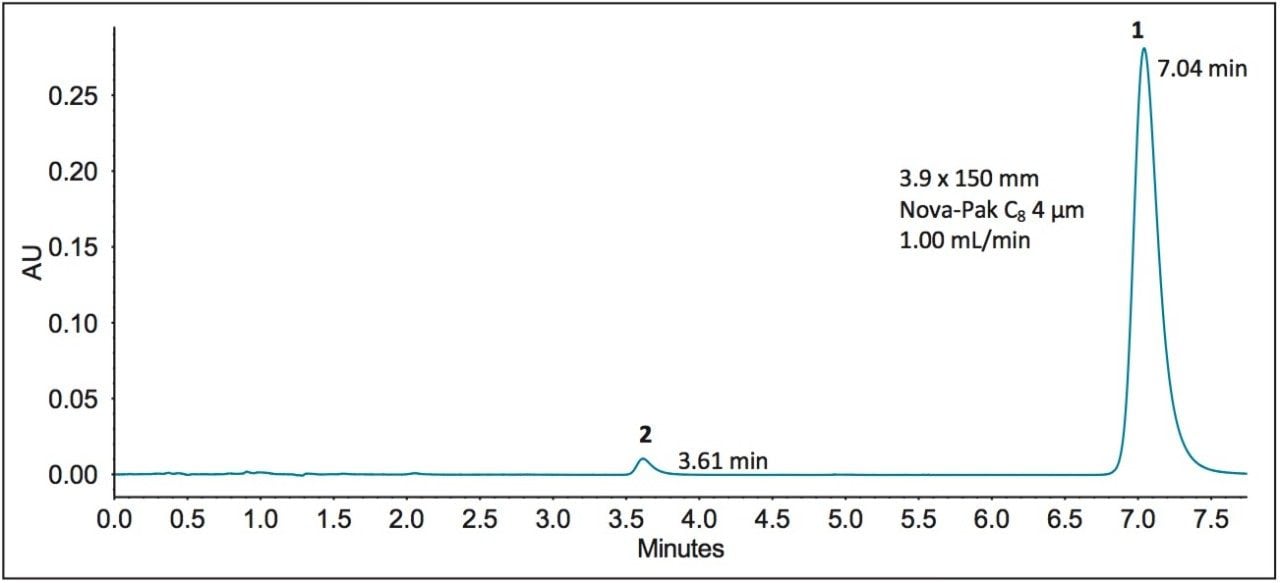
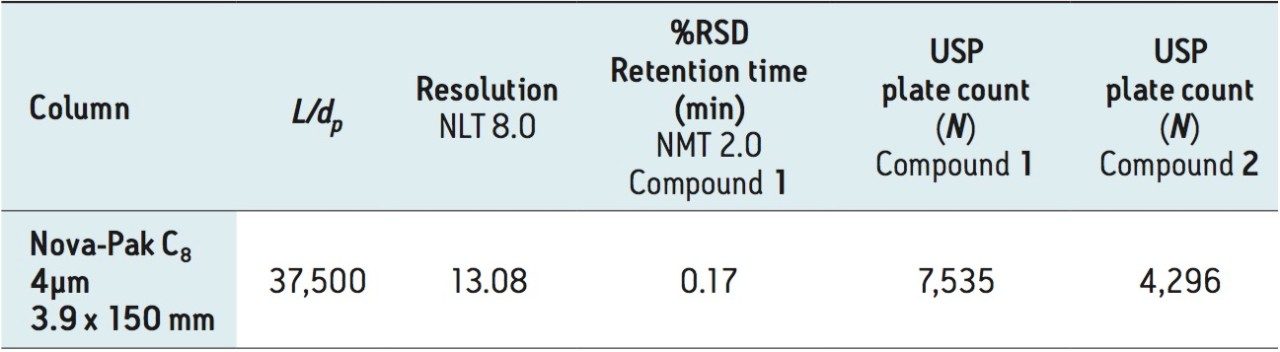
From Table 2, observe that the original column passes the USP method system suitability criteria, which specify that the resolution between the compounds must be not less than (NLT) 8.0 and the %RSD10 for the retention time of dofetilide must be not more than (NMT) 2.0%. Triplicate injections were performed to calculate the %RSD and report the average results.
We first used “equivalent L/dp” from USP General Chapter <621> to modernize the dofetilide USP assay method. Table 2 lists L/dp = 37,500 for the original column in the compendial method. This required use of CORTECS C8 Columns with L/dp between 28,125 (-25%) and 56,250 (+50%). The CORTECS C8 Column, 2.7 μm, 3.0 x 100 mm (p/n 186008361) and the CORTECS UPLC C8 Column, 1.6 μm, 3.0 x 50 mm (p/n 186008409) both satisfy this guideline, with L/dp = 37,037 and L/dp = 31,250, respectively. We thus equipped an ACQUITY UPLC H-Class System with these columns. The flow rates were scaled using eq 4, where “F1 and F2 are the flow rates for the original and modified conditions, respectively; dc1 and dc2 are the respective column diameters; and dp1 and dp2 are the particle sizes.”3
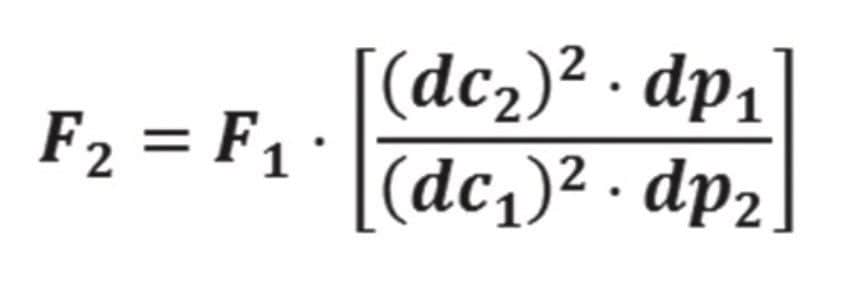
From eq 4, we calculate scaled flow rates of 0.88 mL/min for the CORTECS C8 Column, 2.7 μm, 3.0 x 100 mm (p/n 186008361) and 1.48 mL/min for the CORTECS UPLC C8 Column, 1.6 μm,3.0 x 50 mm (p/n 186008409). Unfortunately,the latter column and flow rate comb ination exceeds the maximum system pressure. USP General Chapter <621> states that isocratic USP method flow rates may be adjusted by ±50% so we reduc ed the flow rate for this column to 1.30 mL/min.
Figure 4 shows the separation on the CORTECS C8, 2.7 μm, 3.0 x 100 mm and the CORTECS UPLC C8, 1.6 μm, 3.0 x 50 mm Columns. For the larger 2.7 μm solid-core particle, dofetilide elutes at 2.33 minutes, which is a 67% decrease in run time and thus a 71% decrease in solvent consumption compared to the compendial method. There is also a large increase in both resolution and efficiency for dofetilide at 90% and 136% respectively.
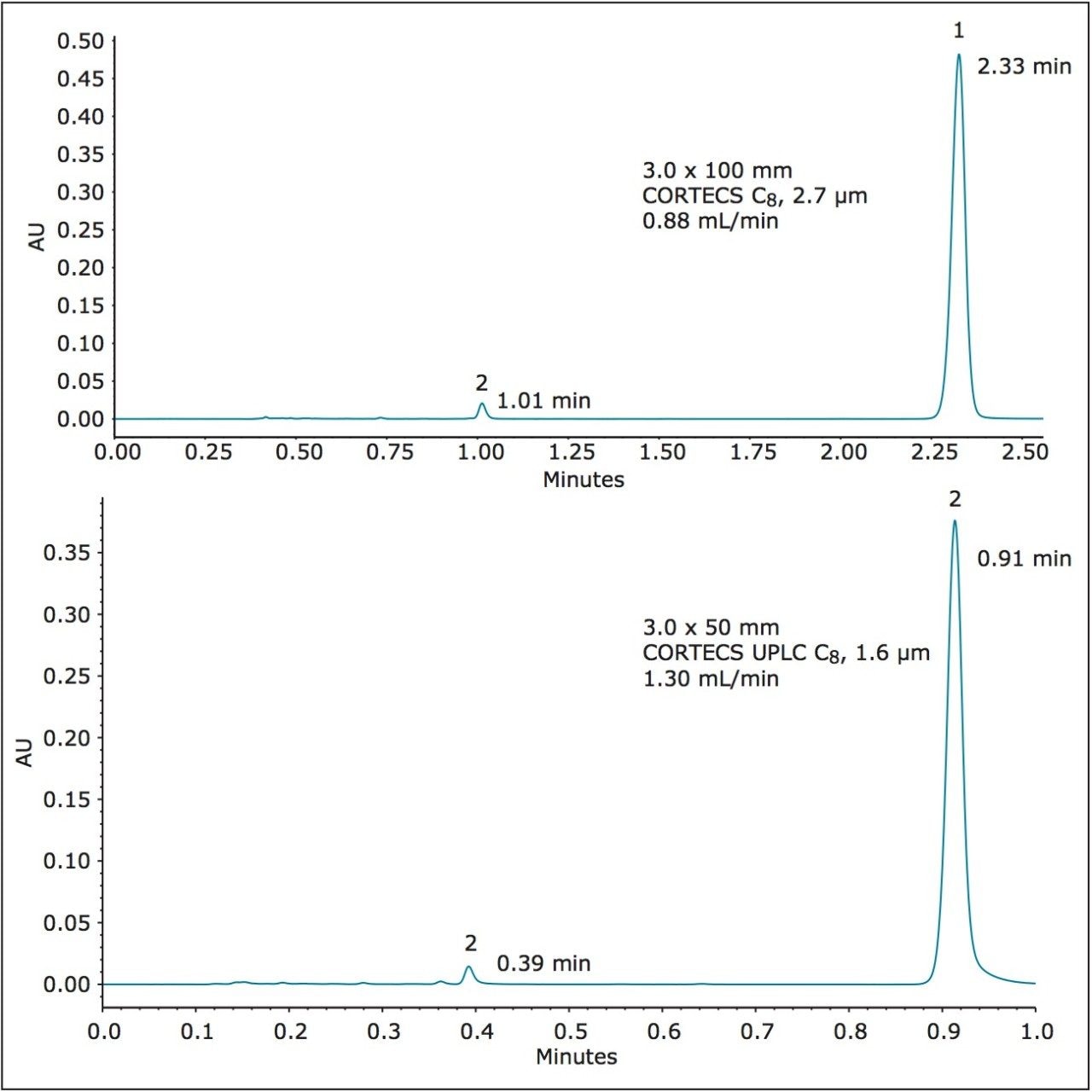
With the sub-2-μm CORTECS Column, the smaller particles allow use of a shorter column resultingin further analysis speed increase and solvent reduction. This is seen by the rapid elution of dofetili de at 0.91 minutes, an 87% run time decrease with still a 74% efficiency increase relative to the original method. There is an associated 83% reduction in solvent consumption. The results of these separations are found in Table 3.
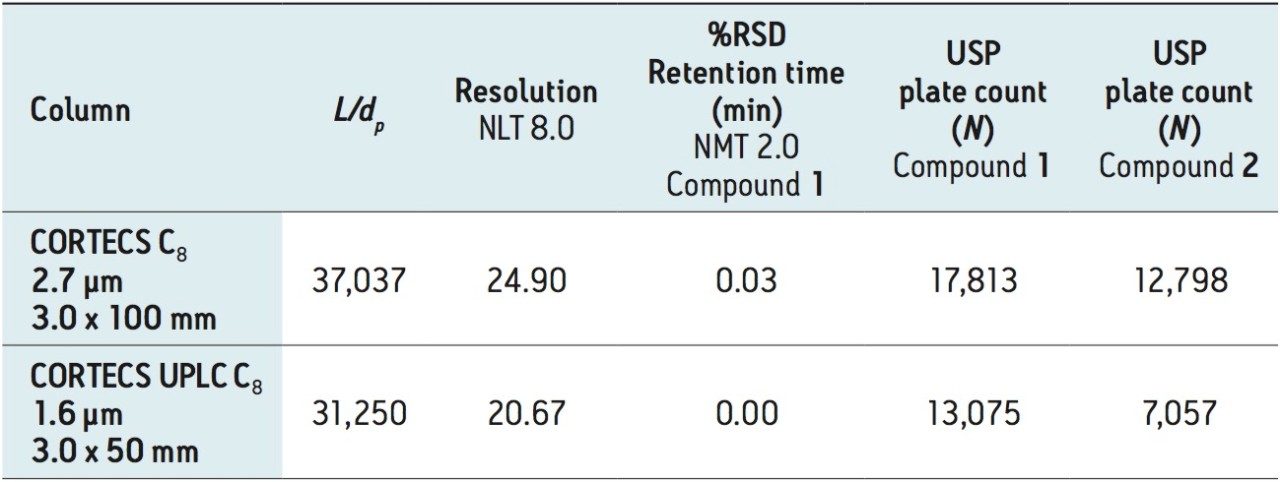
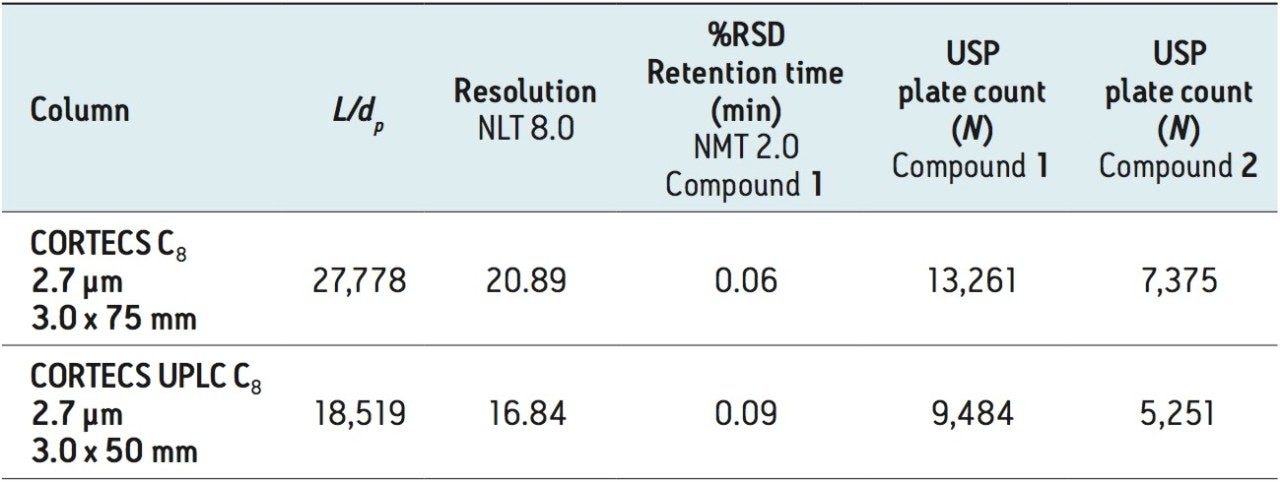
We are at the limit of the modernizations possible using the “equivalent L/dp” allowed changes with the dofetilide USP method. Next, we examined the “equivalent N” guideline. The compendialmethod USP plate count, from Table 2, is N = 7,535 for dofetilide, 1, and N = 4,296 for the rela ted compound, 2. Shorter CORTECS C8 Columns that falloutside of the “equivalent L/dp” range can be used if it can be demonstrated that the measured plate count is in the range of 5,651 (-25%) to 11,302 (+50%) for 1 and 3,222 (-25%) to 6,444 (+50%) for 2.
Since plate count must be experimentally determined, we first equipped the ACQUITY UPLC H-Class System with 2.7 μm CORTECS C8 Columns in the shorter lengths of 3.0 x 75 mm and 3.0 x 50 mm. These column configurations offer L/dp values of 27,778 and 18,519 respectively. Both values lie beyond the lower L/dp limit of 28,125 (-25%) discussed above. In Figure 5 are the chromatograms obtained from these columns.
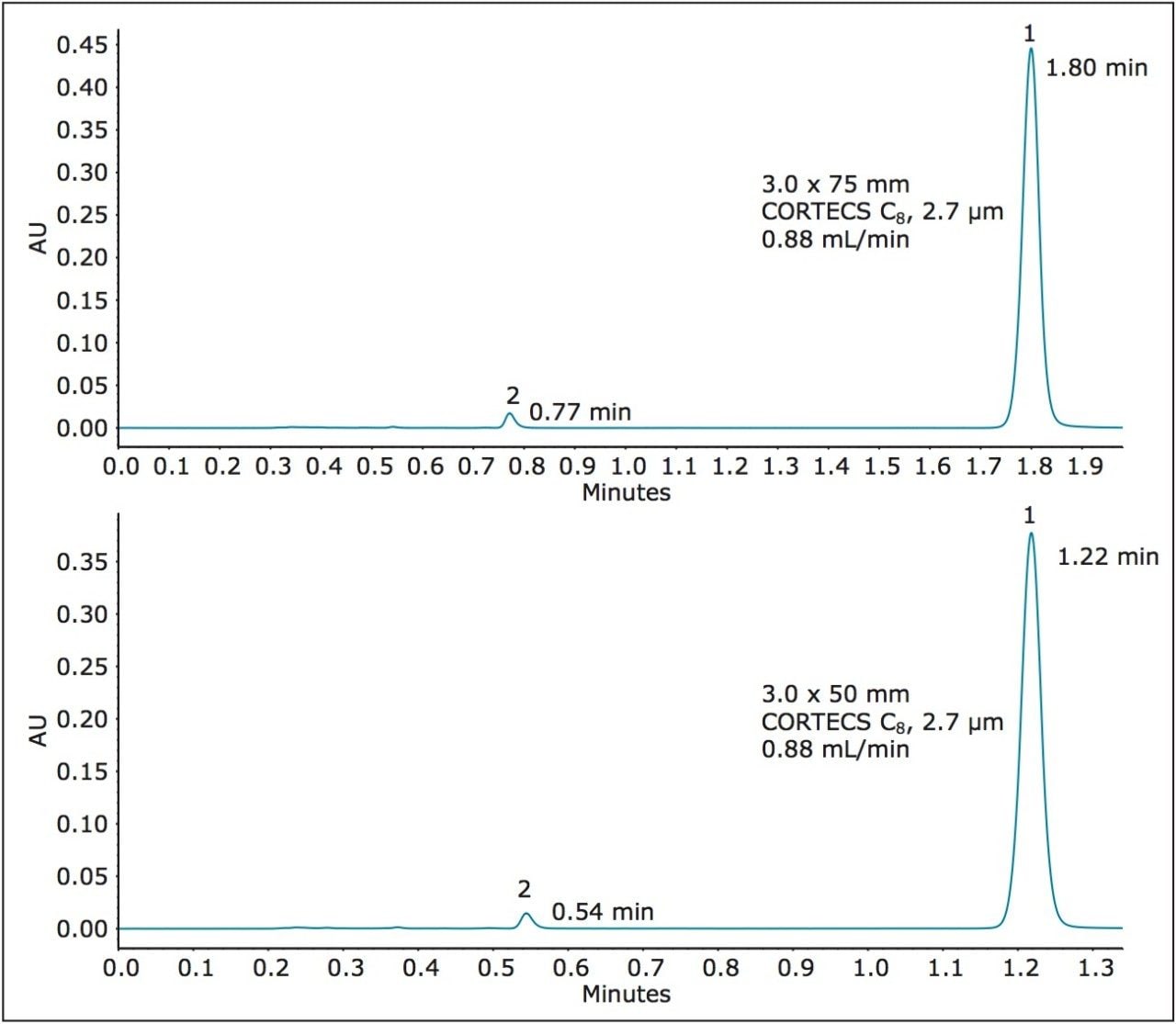
The USP method results for these shorter columns are listed in Table 4. The longer CORTECS C8 Column, 2.7 μm, 3.0 x 75 mm (p/n 186008360) meets all USP method requirements and gives dofetilide a shorter retention time of 1.80 minutes. However, the plate counts for both compounds are outside the upper end of the efficiency range of the original method (+76% for dofetilide, 1, and +72% for the related compound, 2).
Higher efficiency methods are welcomed by analysts. The USP General Chapter <621> cautions that higher efficiency columns may necessitate use of instruments that “minimize extra-column band broadening by factors as instrument plumbing, detector cell volume and sampling rate, and injection volume.”3 The ACQUITY UPLC H-Class System is an example of an UltraPerformance LC instrument with a low dispersion fluid path, a small detector cell volume, and a high detector sampling rate, all designed to handle the reduced peak width and volume caused by modern high efficiency columns such as the CORTECS family. A case could therefore be made that, for UPLC class instruments, efficiencies beyond the +50% guideline are acceptable when modernizing USP methods.
The shorter CORTECS C8, 2.7 μm, 3.0 x 50 mm Column, trades some efficiency to gain still more analysis speed (1 elutes at 1.22 min). This places the resulting modernized method in the USP General Chapter <621> “equivalent N” range while meeting USP method requirements.
A 1.6 μm particle CORTECS UPLC C8 Column in a shorter length was also examined. The CORTECS UPLC C8 Column, 1.6 μm, 3.0 x 30 mm (p/n 186008408) has an L/dp of 18,750, below the “equivalent L/dp” criteria range. When run on the ACQUITY UPLC H-Class System, this column gave the chromatogram in Figure 6.
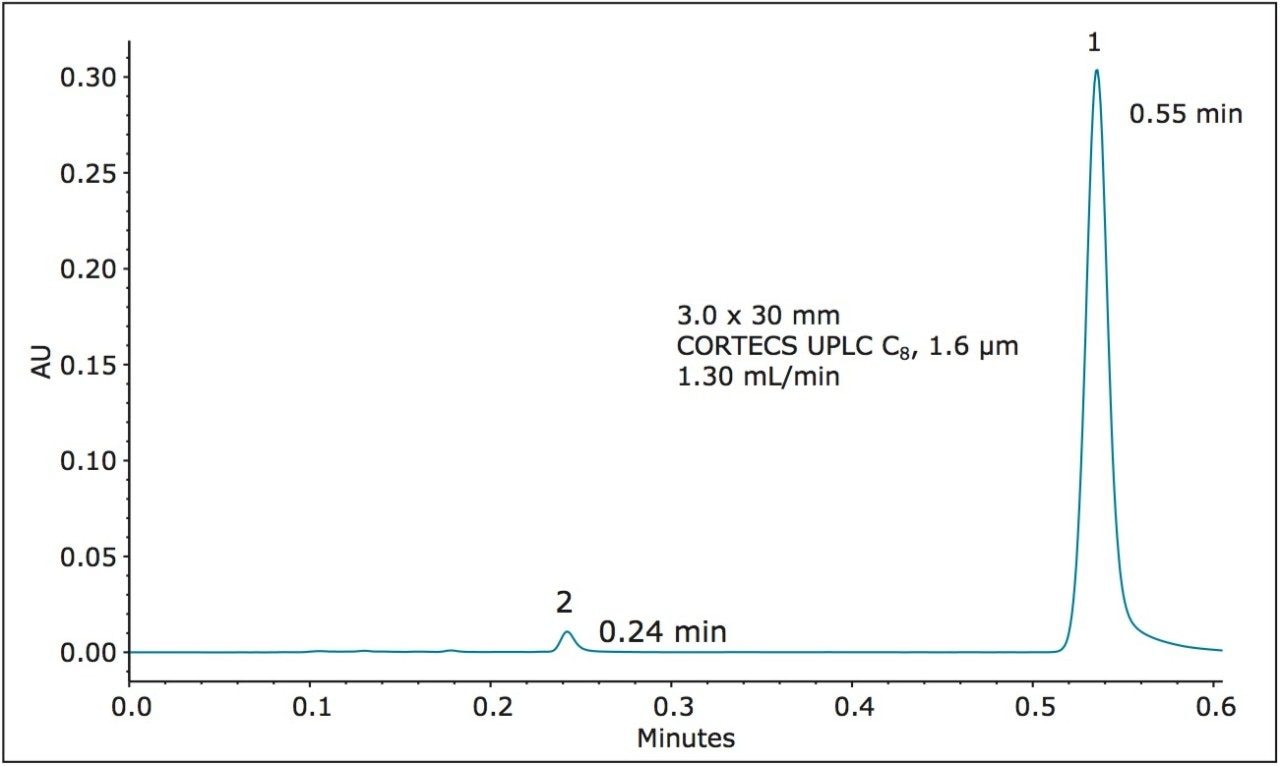
Table 5 summarizes the results. The dofetilide USP method criteria are achieved, the analysis is very fast with dofetilide eluting at 0.55 minutes and the measured plate counts meet the “equivalent N” criteria.
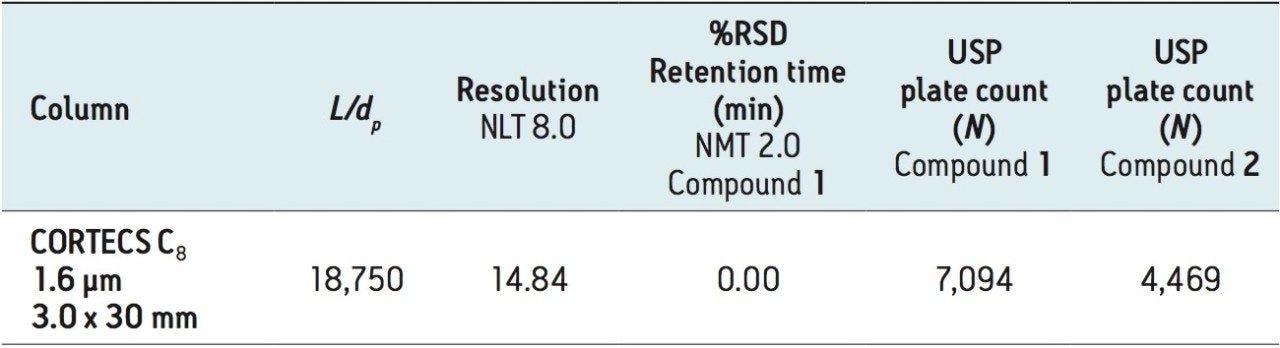
This demonstrates that even the shortest CORTECS UPLC C8, 1.6 μm Column can meet the USP General Chapter <621> “equivalent N ” guideline and also meet the dofetilide USP method requirements. The result is an almost 13 times faster method (92% decrease in analysis time) with a 90% reduction in solvent consumption, compared to the original compendial method. The dramatic decrease in analysis times across all the modernized methods discussed above is summarized in Figure 7.
This application illustrates two approaches for making allowed changes to an isocratic USP method involving modern column particles such as that found in solid-core CORTECS Columns. Using the “equivalent L/dp” guideline is an easy way to modernize a USP method and greatly improve the speed of analysis. With the “equivalent N” guideline, the analyst can realize still further analysis speed improvements from even shorter, highly efficient columns in the CORTECS family.
720005666, April 2016