Simultaneous quantification of DL-amino acids in tea using a robust and sensitive LC-MS/MS method
Abstract
Free amino acids (FAAs) are active compounds in tea which affect the qualities of taste, aroma, and color of the tea. FAA content plays a key role in determining the quality of the tea and is associated with the selling price, with some teas valued up to tens of thousands of dollars per kilogram. D-amino acids are known to be produced under food processing conditions such as fermentation, high temperature or strong acid or alkali treatments. Chiral FAAs may indicate the process and/or storage conditions for the tea product and could be used to grade and price tea through an analytical approach. This application note demonstrates a robust and sensitive UPLC™-MS/MS quantification method for chiral DL-amino acids in tea brew using the Xevo™ TQ-S micro Triple Quadrupole Mass Spectrometer. The analytical method exhibited good linearity for all amino acids, with R2 >0.9934 and residual % <20 %. All enantiomeric pairs were well-resolved on the Daicel ChiralPax ZWIX+ column. With the current method, we were able to detect various D-form amino acids in oolong tea brew, which were on average 10,000-fold lower in concentration as compared to the L-form amino acids.
Benefits
- A robust and sensitive analytical method was developed to accurately quantify 37 DL-amino acids in tea samples using the ACQUITY UPLC I-Class PLUS coupled with the Xevo TQ-S micro Triple Quadrupole Mass Spectrometer
- Separation of all enantiomeric pairs of amino acid were easily achievable on the Daicel ChiralPax ZWIX+ column. This allowed selective and specific identification with accurate quantitation of each isomeric pair with identical MRM transitions
- ACQ derivatization has been proven to improve resolution of DL-amino acids, and boost signal intensity by five to 100-folds compared to underivatized method
Introduction
Tea is a widely consumed beverage and has many important physiological properties and potential health benefits. Free amino acids (FAAs) are key chemicals related to the taste and bioactive quality of tea brew.1 Proteinogenic amino acids, except glycine, form a pair of enantiomers with L- and D-configuration. D- amino acids are naturally present at trace levels and are found in various fermented foods such as vinegar, beer, wine, and tea. In addition, fermentation, high temperature, strong acid, or alkali treatments induce the formation of D-amino acids.3 The study of chiral FAAs may indicate the process and/or storage conditions for the tea product and could be used to grade and price tea through an analytical approach.
FAAs are highly polar with low volatilization and no chromophore, thus the simultaneous determination of FFAs acids in complex commodities, such as tea, is challenging. There are two common analytical approaches based upon high-performance liquid chromatography (HPLC) separation; determination in their native form or in a derivatized form where chemical groups are added, either pre- or post-column, to assist in the analysis. For the determination of FAA enantiomers, a variety of chromatographic methods using chiral derivatizing reagents, chiral mobile phases and chiral stationary phases have been reported. Those chiral columns that can be used with mobile phase systems compatible with mass spectrometry to separate derivatized FFAs, are extremely valuable for the determination of multiple FAAs, including those deficient of chromophores for UV detection. While specific types of derivatizations have been developed for chiral analysis, standard derivatization methods such as aminoquinolyl-N-hydroxysuccinimidyl carbamate (ACQ) have been utilized with chiral columns to allow for enantiomer separation of FFAs. AQC has several advantages as compared with other derivatization reagents, including a simple and well-established derivatization procedure, a shorter derivatization time, fewer side reactions and excellent stability.2 While UV and fluorescence detection are commonly used with ACQ derivatization, tandem quadrupole mass spectrometry (MS/MS) is preferred for the analysis of complex matrices due to its excellent selectivity. Tandem mass spectrometry only requires FAA enantiomers of the same mass (constitutional isomers) and same product ions to be chromatographically separated since overlap of amino acids with different masses does not affect determination.
In this application note, a robust and sensitive method was developed for the quantitation of 37 DL-amino acids in oolong tea brew using an ACQUITY™ UPLC I-Class PLUS System coupled with Xevo TQ-S micro tandem quadrupole mass spectrometer.
Experimental
Calibration Curve Standard Preparation
18 pairs of DL-amino acids and L-glycine were individually dissolved in deionized water (DI) water to a stock concentration of 100 µg/mL each. 2.5 µg/mL chiral amino acids standard mixture was prepared using methanol as diluent. The chiral amino acids mixture was derivatized using AccQ-Tag™ Ultra (p/n: 186003836) according to the protocol in the AccQ-Tag Ultra Derivatization Kit care and use manual.4 Calibration curves were prepared by serial dilution using methanol to 0.25, 1, 2.5, 10, 25, 100, and 250 ng/mL respectively. A set of 25 ng/mL underivatized chiral amino acids standards mixture was also prepared using methanol as diluent.
Tea Sample Preparation
1 g of dry Wenshan Baozhong Oolong tea was weighed and brewed in 50 mL of 100 °C water for ten minutes. The supernatant was filtered, prior to derivatization with AccQ-Tag Ultra. The derivatized tea sample was diluted 10-fold to quantify L-amino acids and the undiluted derivatized tea sample was used to quantify D-amino acids.
Chromatographic Conditions
|
LC system: |
ACQUITY UPLC I-Class PLUS |
|
Column(s): |
Daicel ChiralPak ZWIX+, 3.0 x 150 mm, 3 µm |
|
Column temperature: |
40 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.150 mL/min |
|
Mobile phase A: |
25 mM Ammonium Formate + 0.1% Formic Acid in Methanol: Water (98:2) |
|
Mobile phase B: |
25 mM Ammonium Formate + 0.1% Formic Acid in Water |
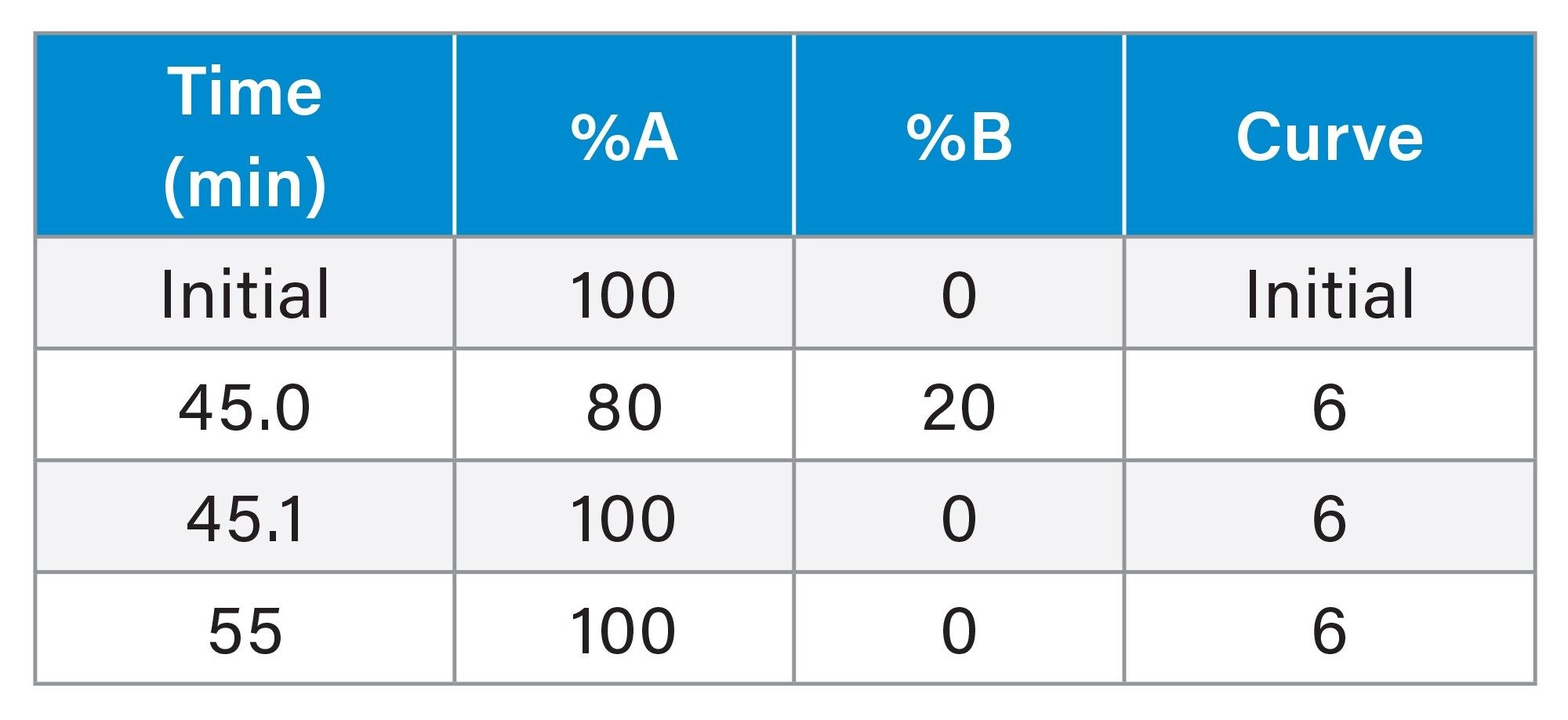
Gradient Table
MS Conditions
|
MS system: |
Xevo TQ-S micro |
|
Ionization mode: |
ESI+ |
|
Desolvation temperature: |
500 °C |
|
Desolvation gas flow (L/Hr): |
650 |
|
Cone gas flow (L/Hr): |
150 |
|
Source temperature: |
120 °C |
|
Cone voltage: |
30 V |
|
Capillary voltage: |
2 kV |
Data Management
|
Chromatographic software: |
MassLynx™ V4.2 |
|
MS software: |
Masslynx V4.2 |
|
Quantitation software: |
TargetLynx™ |
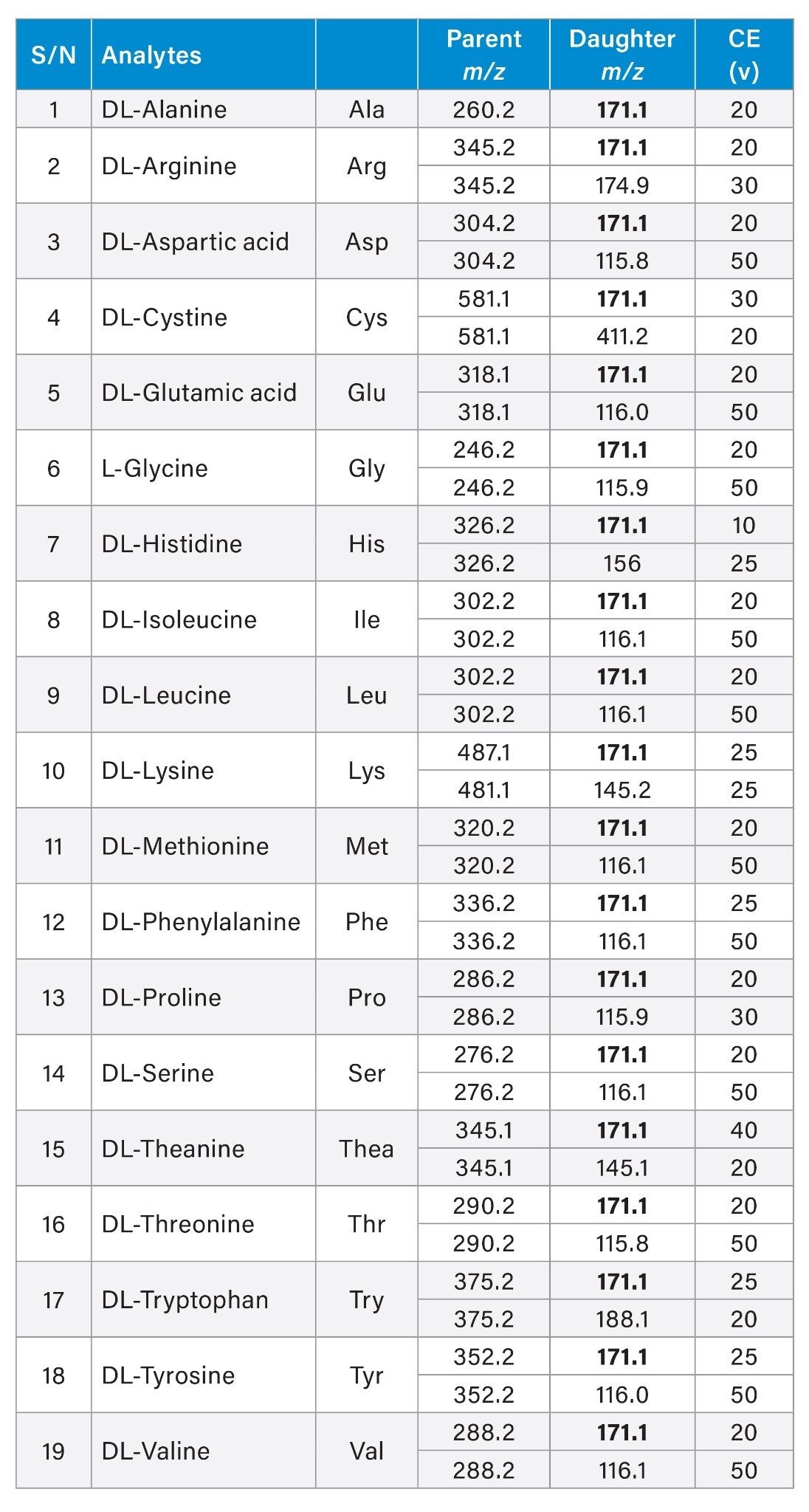
MRM Transitions for Derivatized Amino Acids
Same parameters were used to determine both the derivatized and underivatized amino acids standards and samples. Data was collected using MRM mode, with two transitions for each derivatized chiral amino acid. The MRM transitions and their respective collision energies were optimized using IntelliStart. The selected transitions were the same as those reported in literature.1 Transitions given in bold are the quantifiers in Table 1. During optimization, we observed that derivatized arginine and theanine have identical quantifier transitions. Leucine and isoleucine also have identical MRM transitions as they are structural isomers.
Results and Discussion
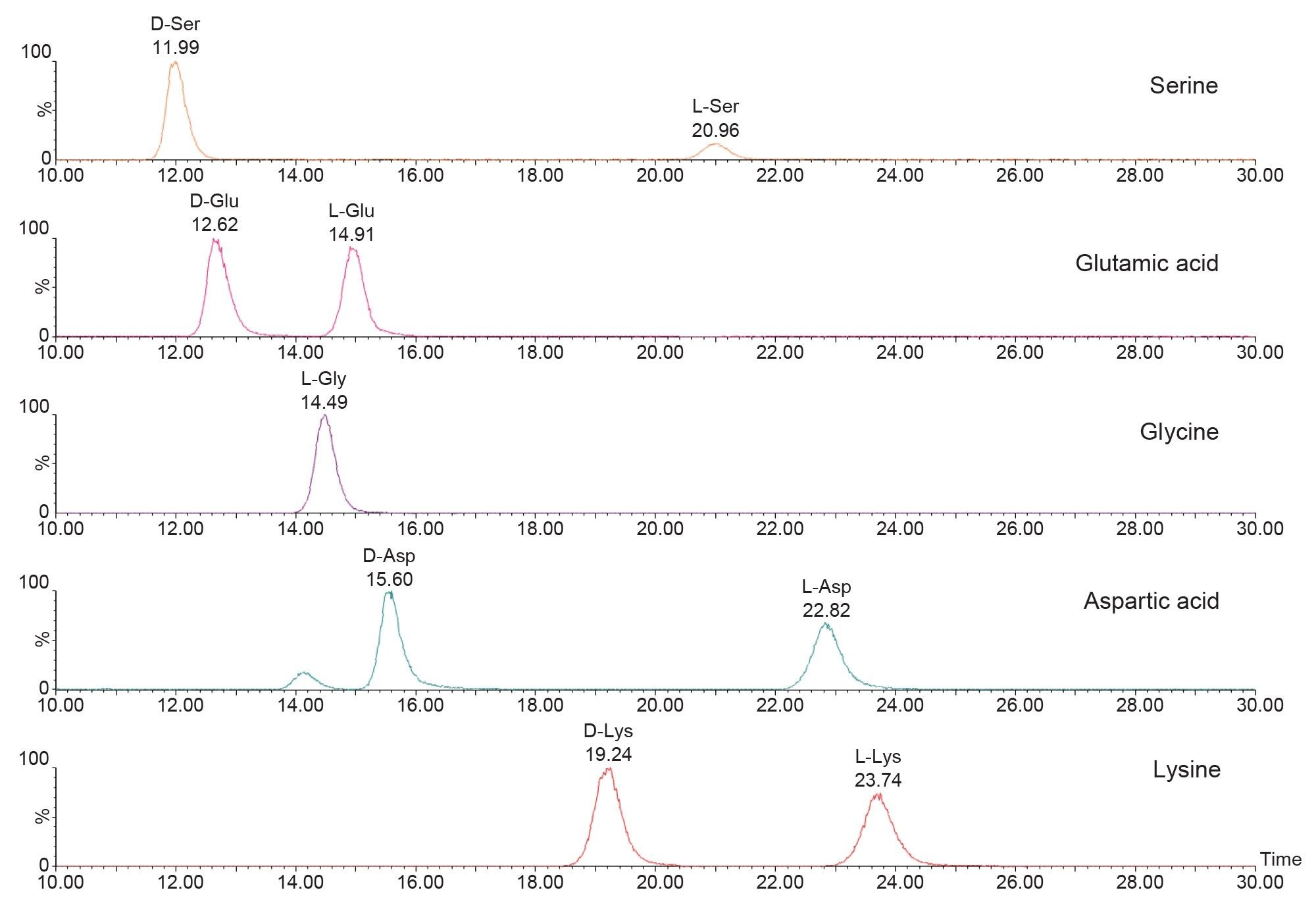
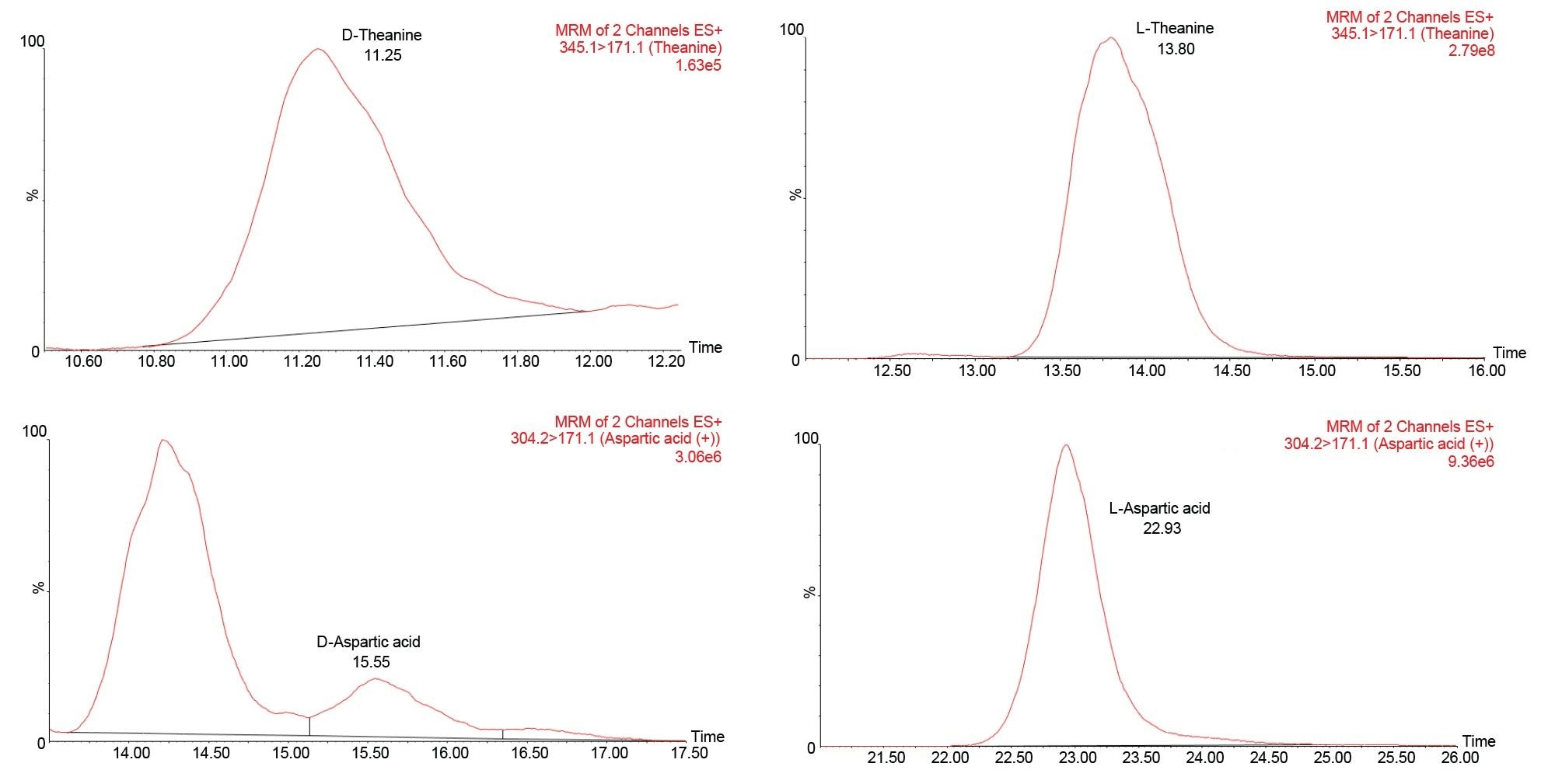
Eighteen pairs of derivatized DL-amino acids together with L-glycine were well separated on the Daicel ChiralPak ZWIX+ column using a 55-minute run time. Analytes with identical quantifier transitions such as isoleucine, leucine, arginine and theanine were resolved. Figure 1 shows the separation of individual DL-amino acid pairs.
Instrument Linearity, Accuracy, Precision and Sensitivity
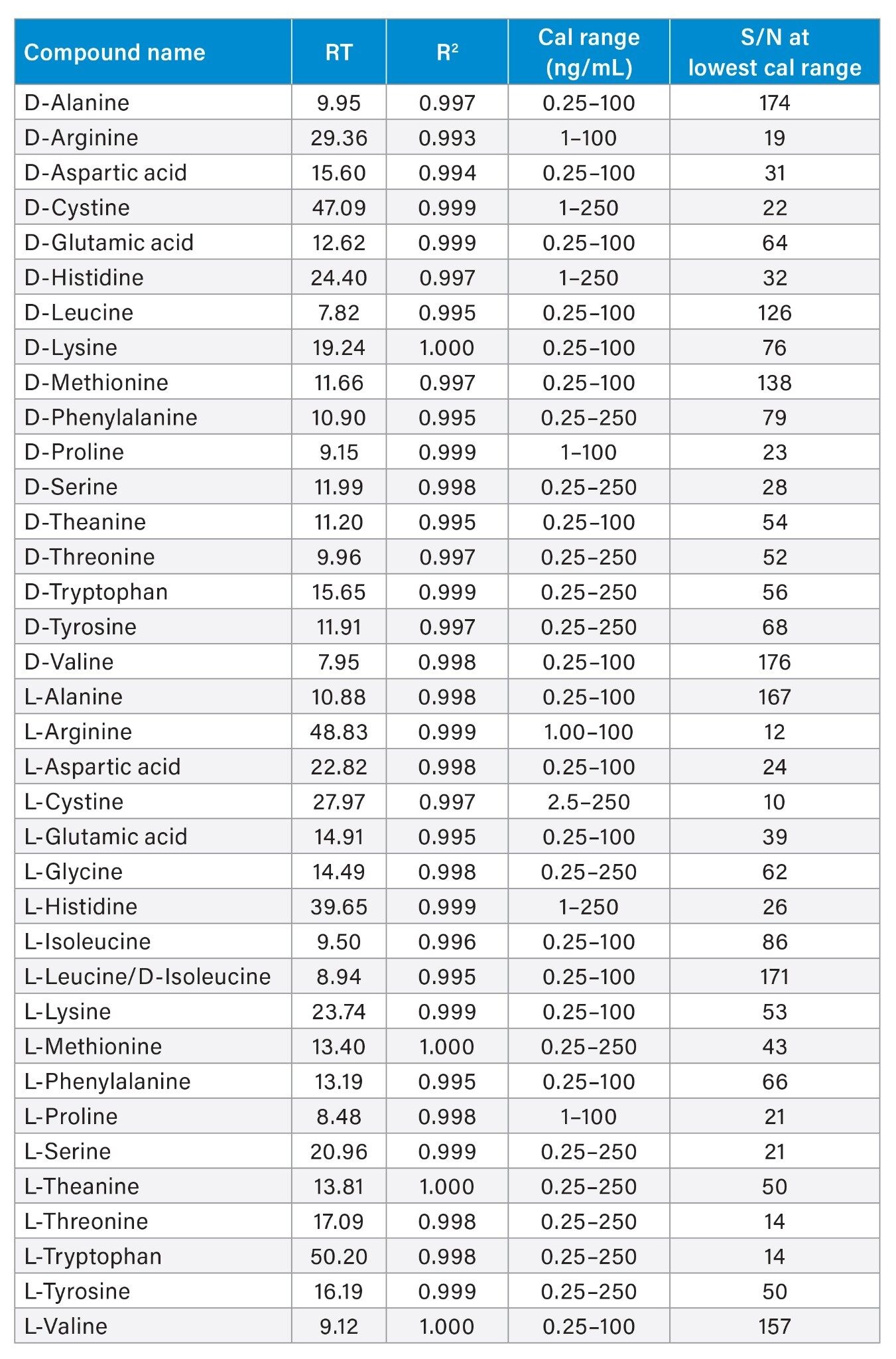
The linear dynamic range for all DL-amino acids is shown in Table 2. The coefficient of determination (R2) achieved for all analytes was greater than 0.993, with residuals <20%. This shows excellent instrument linearity and accuracy for the quantitation of DL-amino acid on the ACQUITY I-Class PLUS System coupled with the Xevo TQ-S micro tandem quadrupole mass spectrometer.
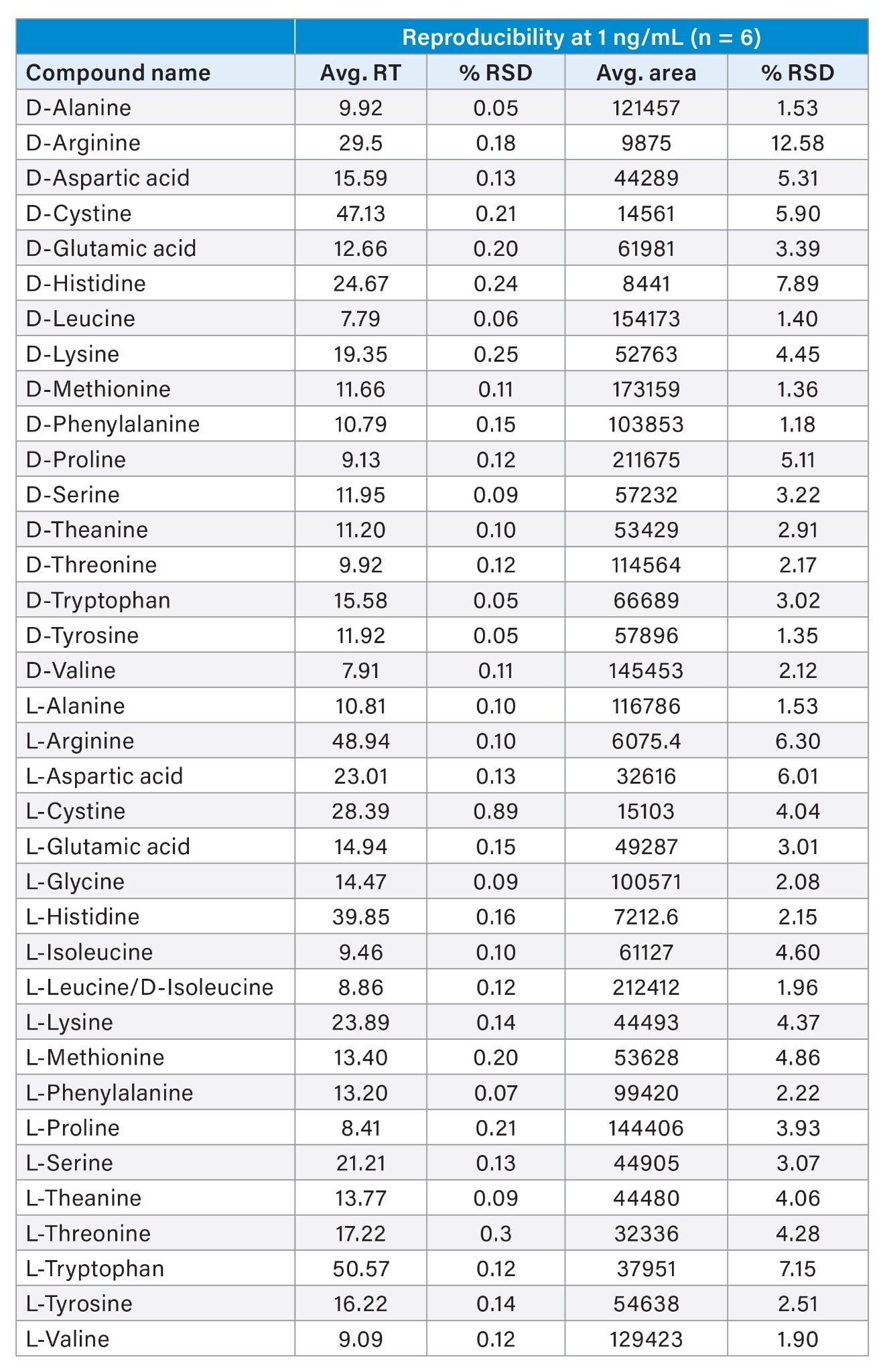
Instrument precision was calculated using the data from six repeated injections at 1 ng/mL. The precision (%RSD) for retention time and peak area at 1 ng/mL was <15%, demonstrating good instrument precision at low concentration. Instrument performance is summarized in Table 2 and 3.
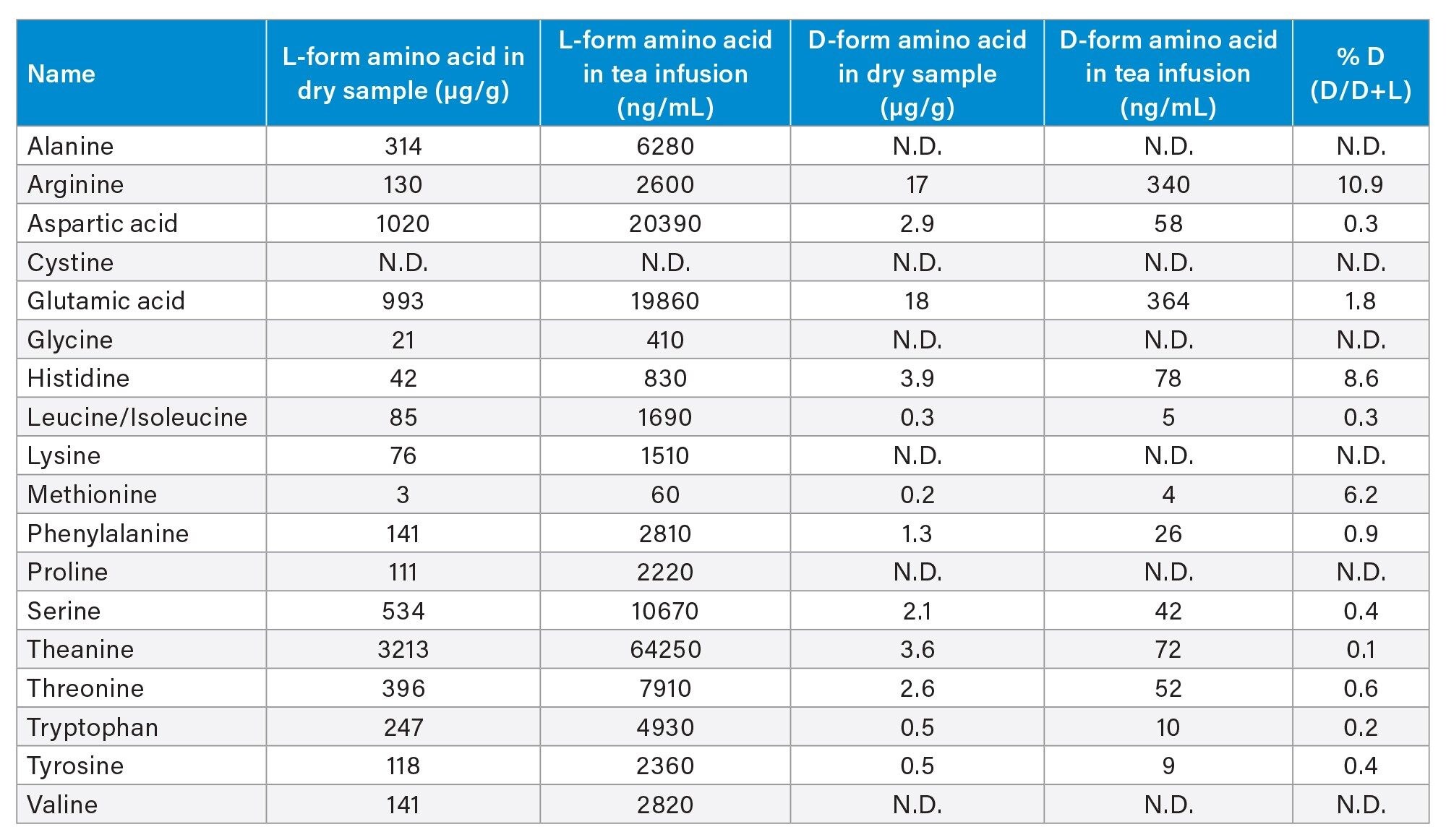
Free L-amino acids were determined in the oolong tea sample after brewing, dilution, and derivatization. The amount of L-amino acids present ranged from 3 to 3000 µg/g in the dry sample, after factoring the dilution due to infusion of the tea leaves. Since the D-form amino acids are reported to be present at trace levels in tea samples, undiluted derivatized oolong tea brew was used for their quantification. Twelve D-amino acids were detected in oolong tea sample, at concentrations ranging from 0.2 to 18.2 µg/g in dry sample. The FFA content for oolong tea brew is summarized in Table 4, and some chromatograms of DL-amino acids detected in tea brew are shown in Figure 2. The % D calculated from our method coincide with literature studying D-amino acid profiles in different types of tea.5 On average, the D-form amino acids were approximately 1,000-fold lower in concentration as compared to L-forms.
Conclusion
Free D-amino acids are naturally present at trace levels in fermented foods such as tea. Different processing conditions affect the content and level of D-amino acids. It is important to have accurate determination of % D content in tea samples due to their direct impact on the product selling price. An MRM method for the determination of 37 DL-amino acids was successfully developed on the ACQUITY I-Class PLUS System coupled with the Xevo TQ-S micro tandem quadrupole mass spectrometer. Chromatographic separation of D- and L- amino acids, after derivatization, was achieved using the Daicel ChiralPax ZWIX+ column. Excellent instrument accuracy, precision and linear dynamic range for DL-amino acids were established in this study. The instrument was also capable of achieving LODs of 0.25 ng/mL for all amino acids except for L-arginine and L-cystine (1 ng/mL). The wide dynamic range of Xevo TQ-S micro allows for direct injection with minimal tea sample preparation to determine D/L amino acid ratio. The method uses a simple and quick approach to sample preparation that reduces the time and cost of the analysis, making this analytical method, established on an ACQUITY I-Class System, fitted with a Daicel chiral column, coupled to a Xevo TQ-S micro tandem quadrupole mass spectrometer, attractive for routine commercial use to determine the D/L amino acid ratio of tea.
References
- Zhou, P., Zhao, F., Chen, M., Ye, N., Lin, Q., Ouyang, L., Cai, X., Meng, P., Gong, X. and Wang, Y. (2019). Determination of 21 free amino acids in 5 types of tea by ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS) using a modified 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) method. J. Food Composition and Analysis, 81, 46–54.
- Castellanos, M., Van Van Eendenburg, C., Gubern, C., Sanchez, J.M. (2016). Ethyl-bridged hybrid column as an efficient alternative for HPLC analysis of plasma amino acids by pre-column derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. J. Chromatogr. B Anal. Technol. Biomed. Life Sci, 1029–1030, 137–144.
- Genchi, G. (2017). An overview of D-amino acids. Amino Acids, 49, 1521–1533.
- AccQ-Tag Ultra derivatization kit care and use manual - 715001331EN.
- Xu, Y., Liu, Z., Liu, Z., Feng, Z., Zhang, L., Wan, X. and Yang, X. (2020) Identification of D-amino acids in tea leaves. Food Chemistry, 317, 126428.
720008166, January 2024