| ACQUITY UPLC I-Class: Assessing Carryover in UPLC/UV Analysis |
| ACQUITY UPLC I-Class: Assessing Carryover in UPLC/UV Analysis |

This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates that the ACQUITY UPLC I-Class System delivers low carryover performance for a diverse range of samples including those at very high concentrations.
The ACQUITY UPLC I-Class System easily manages carryover associated with challenging related compounds’ assays.
One of the greatest challenges of achieving quantification of both high- and low-level components within the same chromatographic separation is managing sample carryover. Often, to see low-level impurities associated with a main analyte, very concentrated samples must be injected. In order to accurately assess the purity of an analyte, it is important to manage the analyte carryover so it does not impact the purity calculation by underestimating the impurities present in a sample. For impurity assays, the sample concentration is high and managing solubility can be challenging. It is important to be attentive to diluent, mobile phase, and wash solvent compositions to achieve low carryover performance for these samples. System design also plays an important role in managing carryover. Often, the simpler the sample introduction into the analysis stream, the easier it is to manage sample carryover, especially when it comes to optimizing the injection method for compounds comprised of very diverse hydrophobicity and polarities.
The ACQUITY UPLC I-Class System was designed to deliver low carryover performance, resulting in the ultimate performance for challenging related compounds’ assays. The Sample Manager’s flow-through needle (FTN) design delivers optimal, high precision injections and provides excellent sample recovery. The inside of the needle is washed during the isocratic run or by the gradient and the exterior of the needle is washed during the chromatographic run.
This allows for excellent management of carryover that does not contribute to the overall inject-to-inject cycle time. To demonstrate the ease of managing carryover on the ACQUITY UPLC I-Class System, three different compounds were selected with very different physical characteristics. Chlorhexidine is ‘sticky’ and typically very difficult to reliably remove from injectors. Dioctyl phthalate is an extremely hydrophobic compound and requires high percentages of acetonitrile to elute it from the column. Caffeine is hydrophilic and is not usually difficult to remove from the injector, making it an excellent probe compound to ensure that no sample is being trapped in the system. Each compound was injected at a concentration that gave a response in the UV at 1.5 AU ± 0.1 AU and the carryover in the subsequent blank injection was measured. No measurable carryover was detected for each of these compounds as shown in Figure 1. To quantify the amount of carryover there was for each of these compounds, highly concentrated samples were prepared (20x to 40x higher concentration) and injected onto the ACQUITY UPLC I-Class FTN. The carryover in the first blank injection for each compound was quantified and determined to be less than 0.001% for each of these very different analytes.
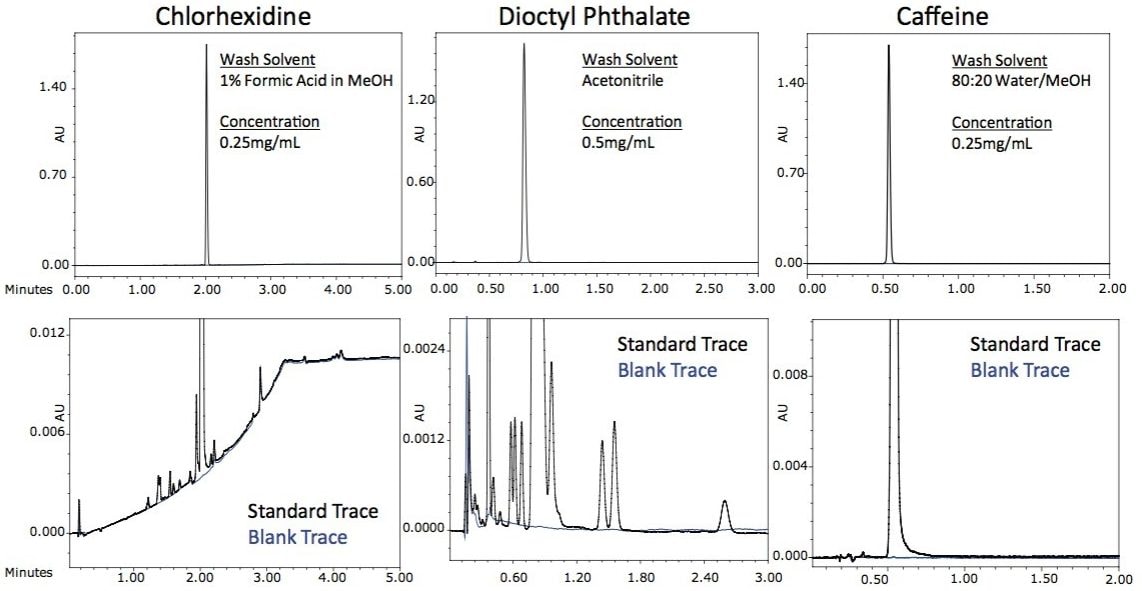
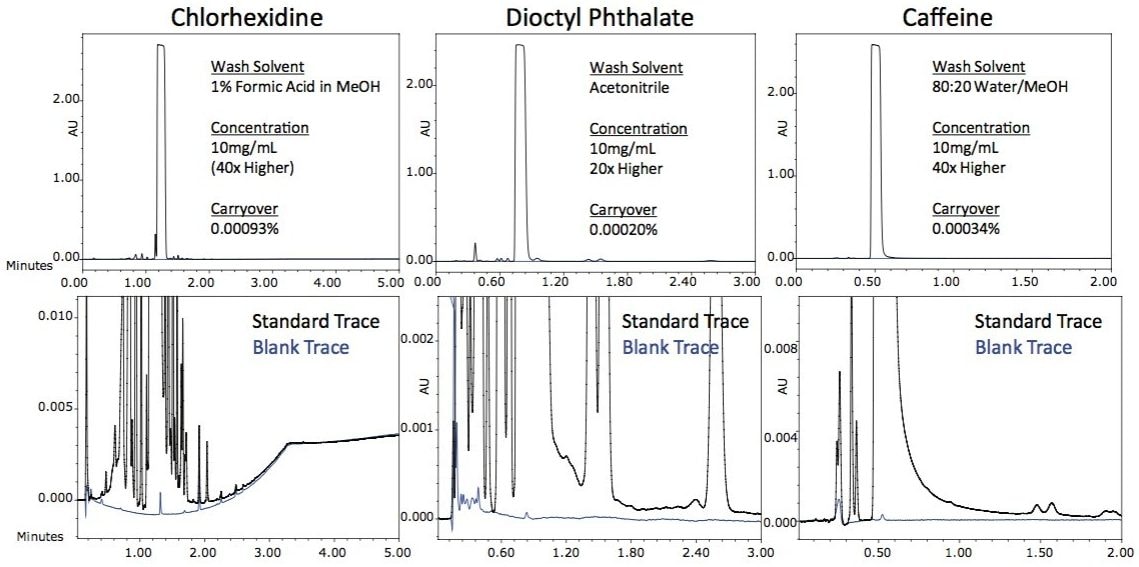
The ability to manage carryover for very diverse analytes is an important attribute of an analytical system. The ACQUITY UPLC I-Class System was designed to deliver excellent carryover performance regardless of the nature of the analyte. The simple and flexible design of the Sample Manager’s FTN injection platform makes optimization of methods simple and intuitive; yielding results required for challenging applications like the analysis of low-level related compounds.
720004137, October 2011