Enhancement of the ETD Product Ion Yield Using Supplemental Activation on the SYNAPT G2 HDMS System
Abstract
To determine the precise location of peptide phosphorylation using ETD.
Benefits
- Obtain unambiguous site-specific assignment of sequence modifications to determine the precise location of peptide phosphorylation.
Introduction
The functionally active form of proteins is often determined not just by the primary amino acid sequence, but by the post-translational modifications that are present. Phosphorylation is challenging to characterize due to low stoichiometry and the transient nature of the modification. In particular, site-specific localization can be confounded by CID as the loss of the phosphate from the peptide backbone is the preferred fragmentation pathway. However, ETD is a powerful fragmentation technique, complementary to CID and has proved particularly useful for the site- specific determination of sites of labile post-translational modifications (PTMs) of peptides and proteins. ETD is a radical-driven fragmentation technique, resulting in cleavage of the peptide N-Cα bond to give c and z• peptide product ions (cf. b and y” ions using CID); it allows precise localization of sites of phosphorylation. Peptides/proteins are dissociated by electron transfer from a donor chemical anion, to positively charged cations, which leads to an inherently similar fragmentation process to electron capture dissociation (ECD).
Results and Discussion
A nanoACQUITY UPLC® System was utilized to optimize the peptide separation on an ACQUITY UPLC® 1.8 µM HSS T3 75 µM x 150 mm Column, using a gradient from 1% to 40% acetonitrile + 0.1% formic acid over 30 min at a flow rate of 300 nL/min. The UPLC® eluent was passed directly to the NanoFlow™ Ion Source of the mass spectrometer. A typical chromatogram showing the tryptic peptide separation is shown in Figure 1.
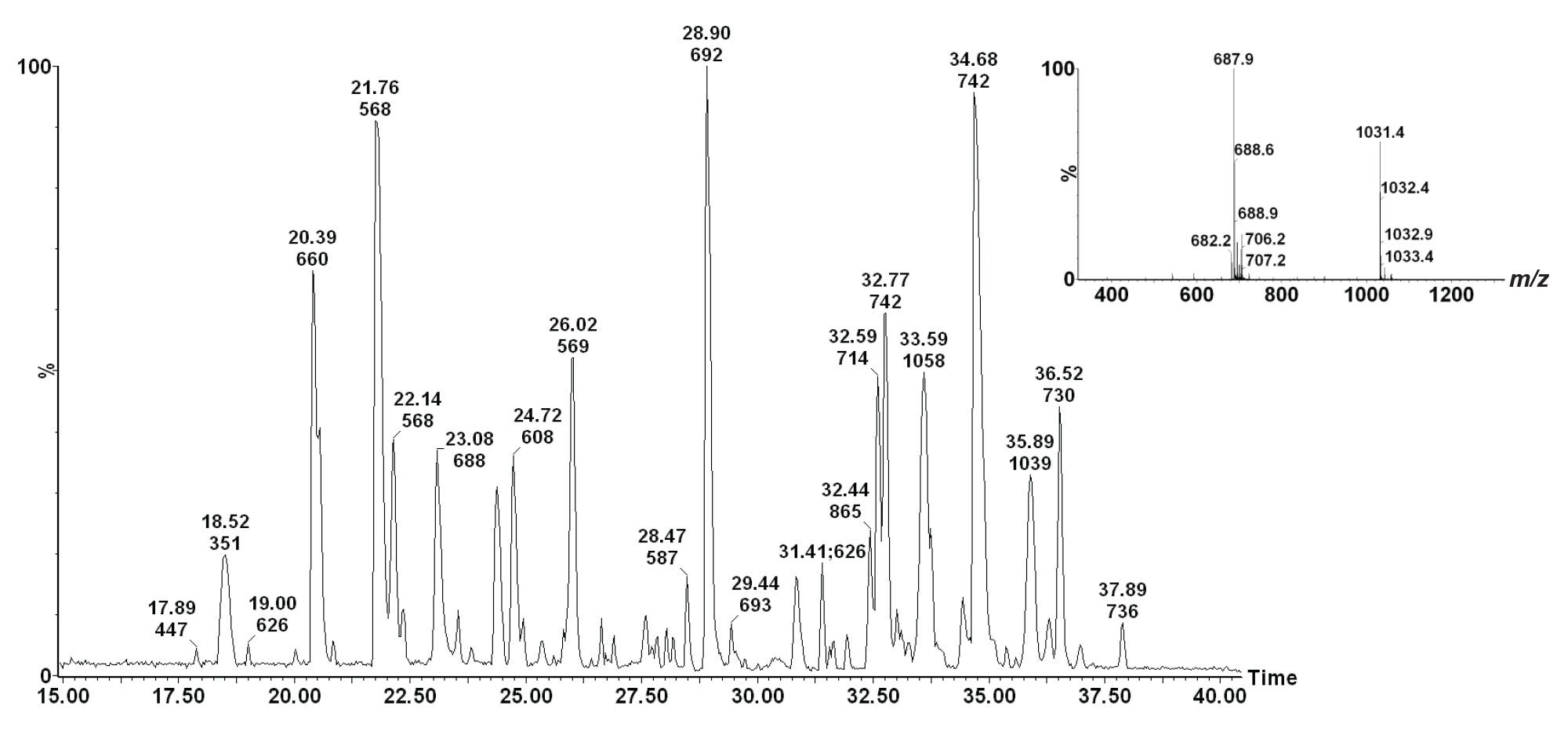
ETD experiments were performed on the SYNAPT® G2 HDMS System. The NanoFlow ESI Source incorporates a fast and efficient intermediate pressure glow discharge reagent anion source. During the course of the ETD experiment, cations and anions are sequentially generated. The ion source block temperature was set at 120 °C. The ion source polarity and the quadrupole set mass were sequentially switched to deliver triply-charged precursor cations, and singly-charged radical anions formed from 1,4-dicyanobenzene (m/z 128) into the first (Trap) T-Wave™ where they interact to form ETD product ions.
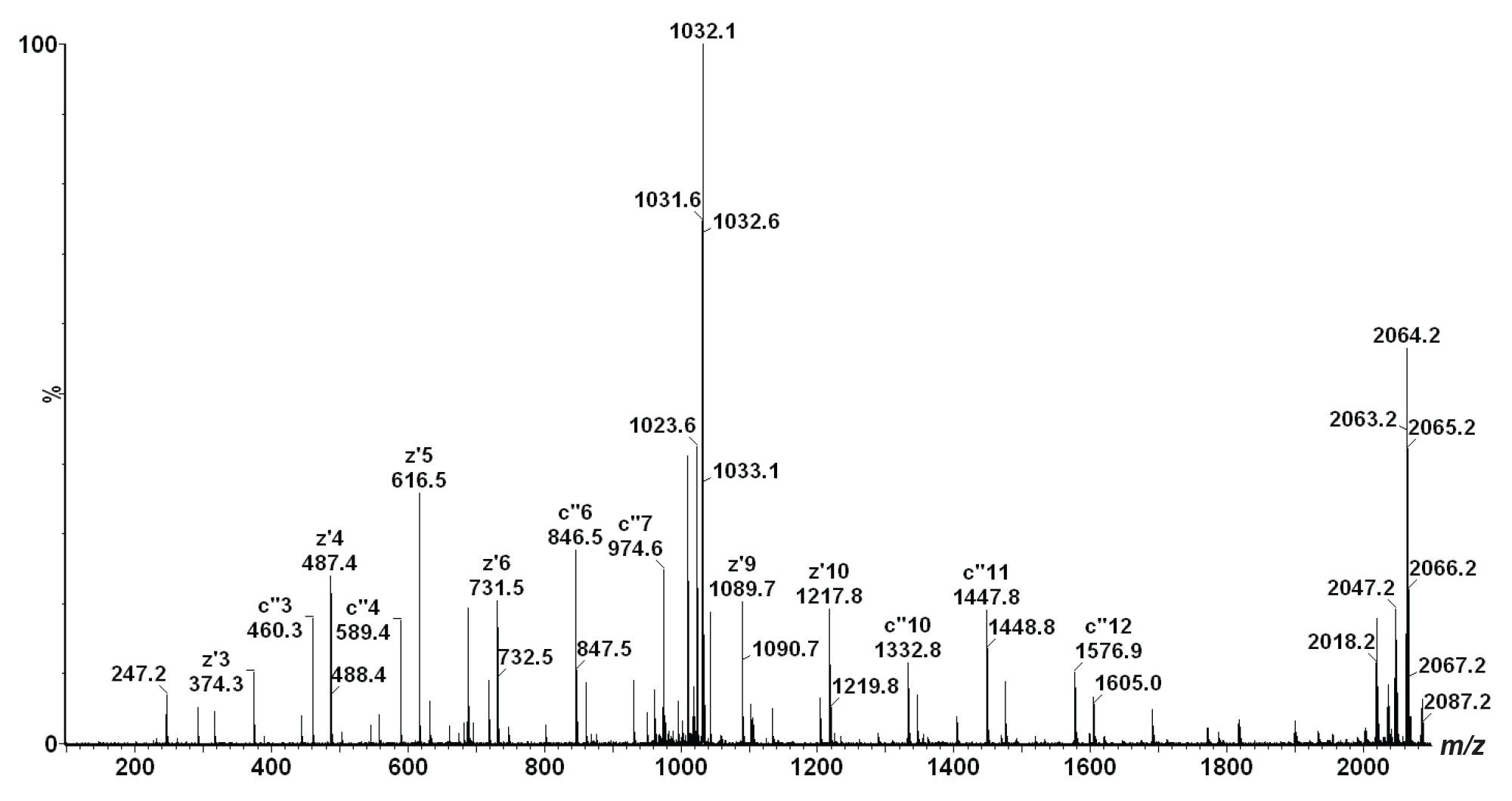
Figure 2 shows the ETD mass spectrum of the triply charged phosphopeptide of m/z 688. The mass spectra show no magnification of the m/z scale. This spectrum was obtained by slightly increasing the ions kinetic energy (10 eV supplemental activation (SA)) as they enter the Transfer T-Wave. N-terminal, c ions were detected for c2+- c15+ together with C-terminal, z ions for z2+• - z15+• in the ETD spectrum. The supplemental activation increased the ETD product ion yield across the m/z range, and allowed unambiguous site-specific assignment of the sequence modification.
Conclusion
In this technology brief, we have demonstrated that the nanoACQUITY UPLC System combined with ETD allows the unambiguous site-specific assignment of sequence modifications. ETD confirmed that the serine residue and not the threonine residue has been phosphorylated (as expected, since the phosphorylation site is known).
720003926, August 2022