In this application note we use a RAC/DMRAC mixture as an example to demonstrate the use of preparative SFC for the rapid purification of raclopride. The advantages of SFC for purifying radiolabeled imaging agents are discussed, including speed, fast dry down, and the ability to alter the elution order for high purity compound recovery.
Positron Emission Tomography (PET) is an imaging technique for the diagnosis and treatment of a variety of diseases, as well as for drug development. The imaging scans involve the use of radioactive tracer materials containing 11C, 15O, 13N, or 18F, all with relatively short half lives. To minimize the decay loss, the radiotracers need to be synthesized, purified, and sterilized within two to three half lives before being administered to the human or animal subject.
Ralcopride is a commonly used PET ligand for studying the D2 receptor system in the brain.1 The positron emitting isotope 11C (t1/2 = 20.4 min) is typically incorporated into the raclopride (RAC) molecule via methylation of its precursor desmethyl ralcopride (DMRAC), as shown in Figure 1. Currently, raclopride is purified by preparative reverse phase liquid chromatography (RPLC).2-4
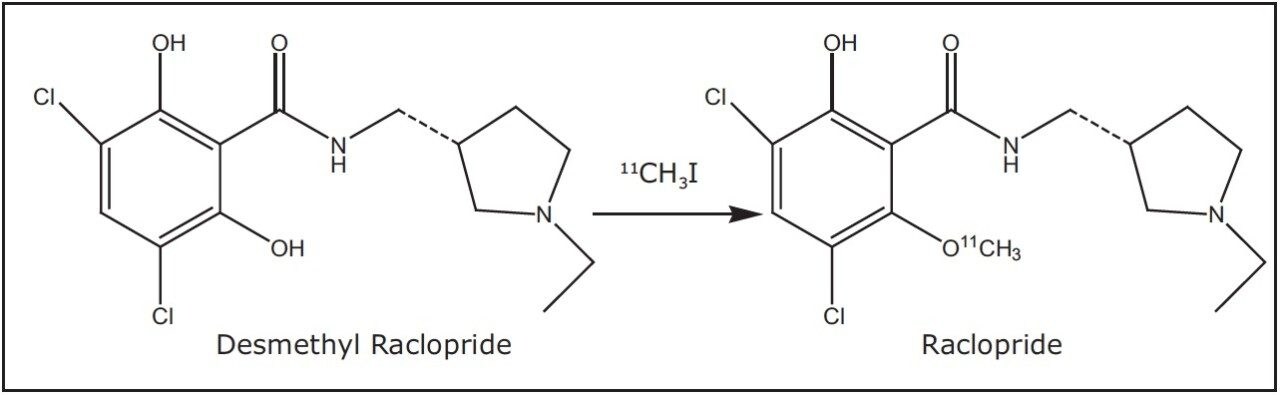
Herein, we use a RAC/DMRAC mixture as an example to demonstrate the use of preparative SFC for the rapid purification of raclopride. The advantages of SFC for purifying radiolabeled imaging agents are discussed, including speed, fast dry down, and the ability to alter the elution order for high purity compound recovery.
|
Co-solvent: |
Methanol with 3% (v/v) 7N NH3/methanol |
|
Temp.: |
40 °C |
|
Back pressure: |
120 bar |
|
Injection vol.: |
10 μL |
|
Flow rate: |
3.0 mL/min |
|
PDA/UV: |
220 to 300 nm |
|
Isocratic: |
30% |
|
Columns: |
Diol, Propylpyridyl Urea (PPU), Cyano and Phenyl (4.6 x 150 mm, 5 μm) |
|
Co-solvent: |
Methanol with 3% (v/v) 7N NH3/methanol |
|
Temp.: |
40 °C |
|
Back pressure: |
120 bar |
|
Injection vol.: |
80 μL |
|
Flow rate: |
8.6 mL/min |
|
PDA/UV: |
239 nm |
|
Isocratic: |
22% |
|
Column: |
Cyano (7.8 x 75 mm, 5 μm) |
Analytical method development was done on a Waters Resolution SFC MS System controlled by MassLynx Software. The system consists of the following components: Fluid Delivery Module (FDM), Automated Back Pressure Regulator (ABPR), Alias Autosampler, 10-port Analytical-2-Prep Column Oven, 2998 Photodiode Array (PDA) Detector, and 3100 MS Detector.
All preparative experiments were performed on an Investigator SFC System controlled by ChromScope Software. The system consists of the following components: FDM, ABPR, Alias Autosampler, 10-port Analytical-2-Prep Column Oven, 2998 PDA Detector, make-up pump, and six-position Fraction Collection Module.
Raclopride and desmethyl raclopride were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade methanol and NH3/methanol solution were purchased from Thermo Fisher Scientific (Fair Lawn, NJ, USA).
Alkylation of a non-radioactive precursor is a common synthetic route to incorporate the radiolabels into the radiotracers, as in the case of the synthesis of raclopride shown in Figure 1. As a result, the radiolabeled final product is more hydrophobic than the precursor. For example, the LogPs for desmethyl raclopride (precursor) and raclopride (product) are 1.93 and 2.20, respectively. Furthermore, in order to drive the completion of the limiting radiolabel agent, a large excess of precursor is often used. The resulting crude reaction mixture, therefore, contains a relatively low quantity of radioactive product in a large excess of un-reacted precursor.
With a classic C18 column in RPLC, desmethyl raclopride elutes off the column before raclopride. However, due to the wide dynamic range of the crude mixture, there is a high likelihood that desmethyl raclopride tails into the raclopride peak; thus, contaminating the final formulation. The issue is further compounded by the time constraint imposed by the short half life of 11C. It is, therefore, highly desirable to have raclopride elute off the column before desmethyl raclopride. Obviously, this can be achieved by normal phase liquid chromatography (NPLC), but the use of toxic organic solvents in NPLC is prohibitive for its adoption in such purification. Hydrophilic interaction chromatography (HILIC) was attempted, but there was no success for the RAC/DMRAC mixture.3 Gillings et al. demonstrated the elution order reversal in RPLC by using a bonded C16 alkylamide column in RPLC to induce stronger polar interactions. The total chromatography time was 20 min.4
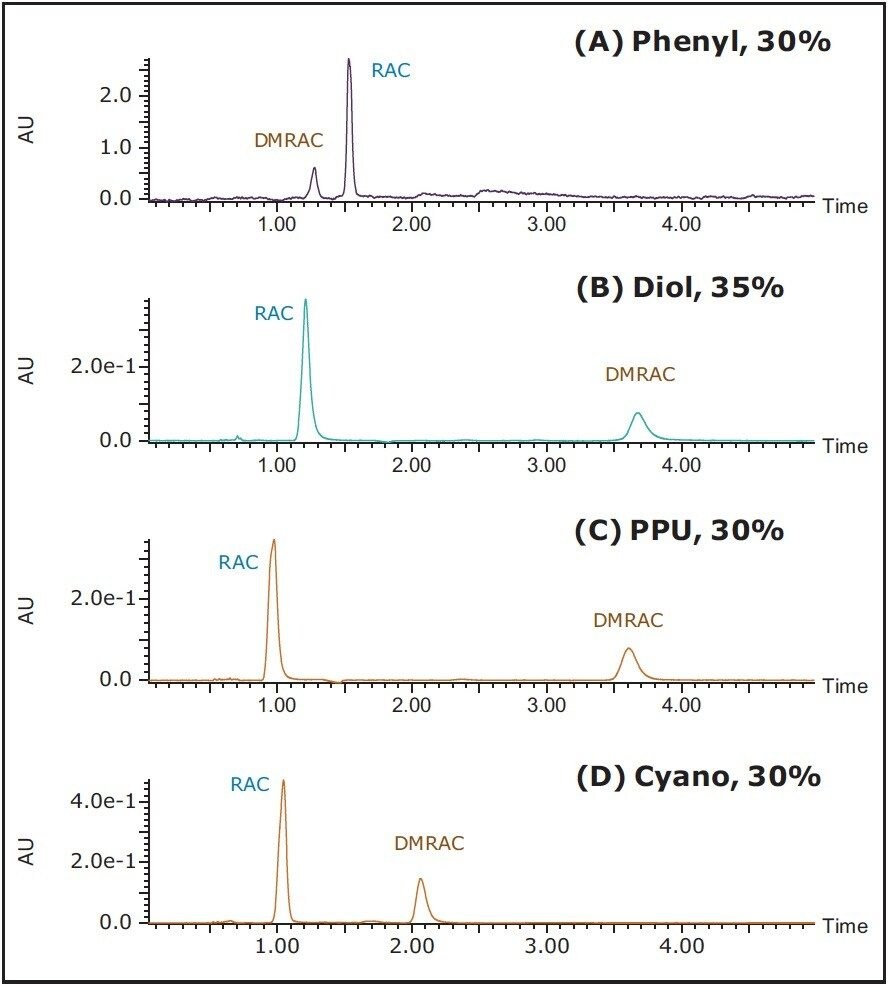
To that end, SFC has the potential to resolve all aforementioned challenges. By primarily using CO2 and alcohols as the mobile phase, SFC eliminates the use of toxic organic solvents and is deemed a “greener” normal phase chromatography. SFC has also proven advantageous in speed and fast dry down in purification. Figure 2 shows the SFC/UV chromatograms of the RAC/DMRAC mixture using four different columns under isocratic conditions. The peaks were identified using MS (results not shown).
Clearly, all four columns yielded baseline resolutions between RAC and DMRAC in less than 5 min. The elution order varied depending on the stationary phases. When a phenyl column was used, shown in Figure 2A, the elution order is the same as those observed with classic RPLC.2 However, when polar stationary phases were used – such as diol, PPU, and Cyano, shown in Figure 2B-D – all three chromatograms exhibited the desired elution order, i.e., target compound before the precursor. The stationary phases used in SFC encompass both polar and non-polar phases, offering a great deal of flexibility in altering the elution order of the analytes.
Based on the analytical runs, the Cyano column was selected for scale-up purifications, due to shorter run time and relatively lower co-solvent percentage. To further reduce the preparative run time, a short Cyano column (75 mm in length) was used, and the co-solvent percentage was adjusted accordingly. Figure 3 is a representative chromatogram for the purification of a RAC/DMRAC mixture. Ensuing fraction analyses indicated 100% purity for both fractions. It is noteworthy that NH3/methanol was used as the additive in the mobile phase based on its lower toxicity and higher volatility.
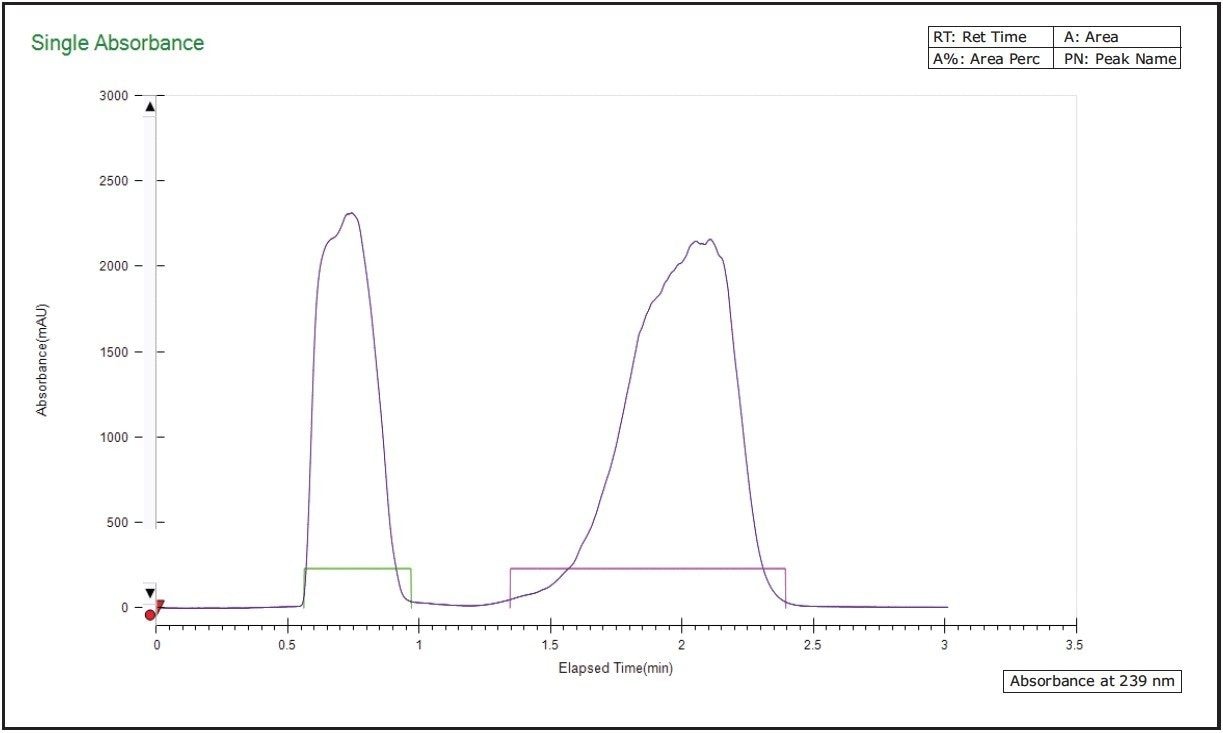
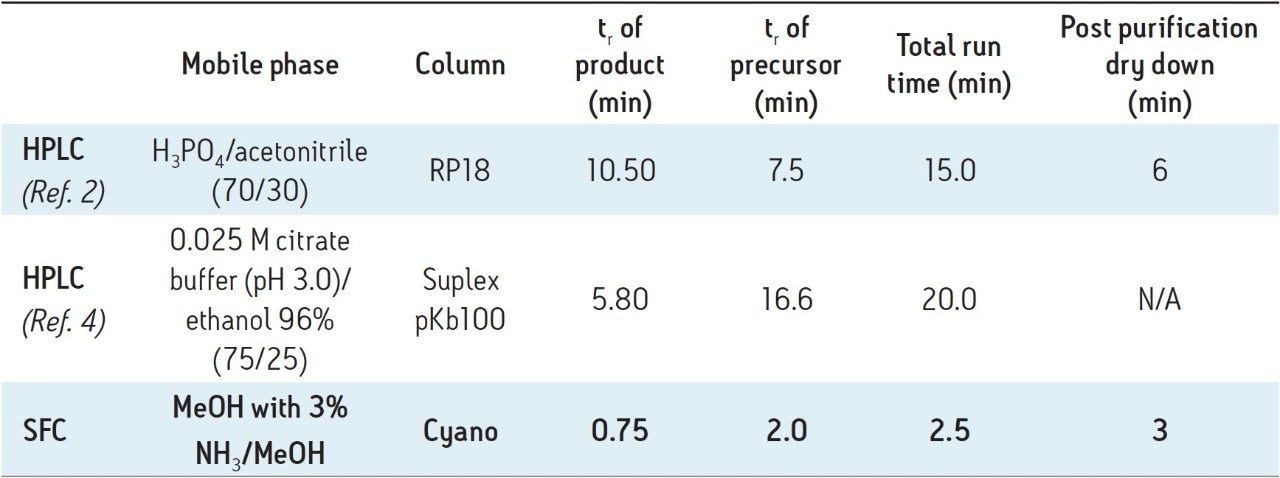
Table 1 illustrates a comparison between the SFC method and two reported HPLC methods. Clearly, the SFC method offers the shortest run time. In addition, post purification dry down was shorter because fractions were collected in a small volume of volatile methanol; whereas, in the two HPLC methods, a majority of the mobile phase (>70%) was aqueous buffer. Compared to the two HPLC purifications, the overall process time using SFC was substantially shorter, which can lead to a significant gain in the final yield of the valuable 11C-labeled product.
Raclopride can be readily separated from its precursor, desmethyl raclopride, in the desired elution order, on a variety of stationary phases using SFC. SFC offers a wide range of selectivity, which allows for easy maneuvering of the elution order of the analytes. This is particularly useful for the purification of low concentration analytes in a mixture of wide dynamic range. For raclopride/desmethyl raclopride purification, the described SFC method also provided shorter overall process time, including both chromatography and post purification dry down, critical for the purification of radiolabeled agents with short half lives. As SFC continues to make inroads in pharmaceutical and associated industries as a viable chromatographic technique for purification, it also holds great promise to become an integral contributor to radio-pharmaceutical research.
720004207, January 2012