
In this application note, we have developed a 15 minutes method for quantitative analysis of PE and PC on the ACQUITY UPLC H-Class Plus System with a PDA Detector.
Quantification of PE and PC in rice and sunflower oil lecithin within 15 minutes run time on the ACQUITY UPLC H-Class Plus System with a PDA Detector.
Phospholipids are major constituents of cell membrane and are found in all tissues and subcellular compartments as mixtures of various molecular species such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), sphingomyelin (SM), and lysophosphatidylcholine (LPC) depending on the type of polar head groups and the degree of unsaturation of the acyl chains. Among these phospholipids, PC and PE represents a major constituent of cell membranes. The demand for lecithin with high PC and PE content from vegetable or cereal source is increasing these days, particularly in pharmaceutical, cosmetic, food, and other applications due to their emulsifying properties and nonantigenic nature. The application of lecithins in pharmaceutical and cosmetics domain depends mainly on the PC and PE with its saturated or unsaturated fatty acid content.
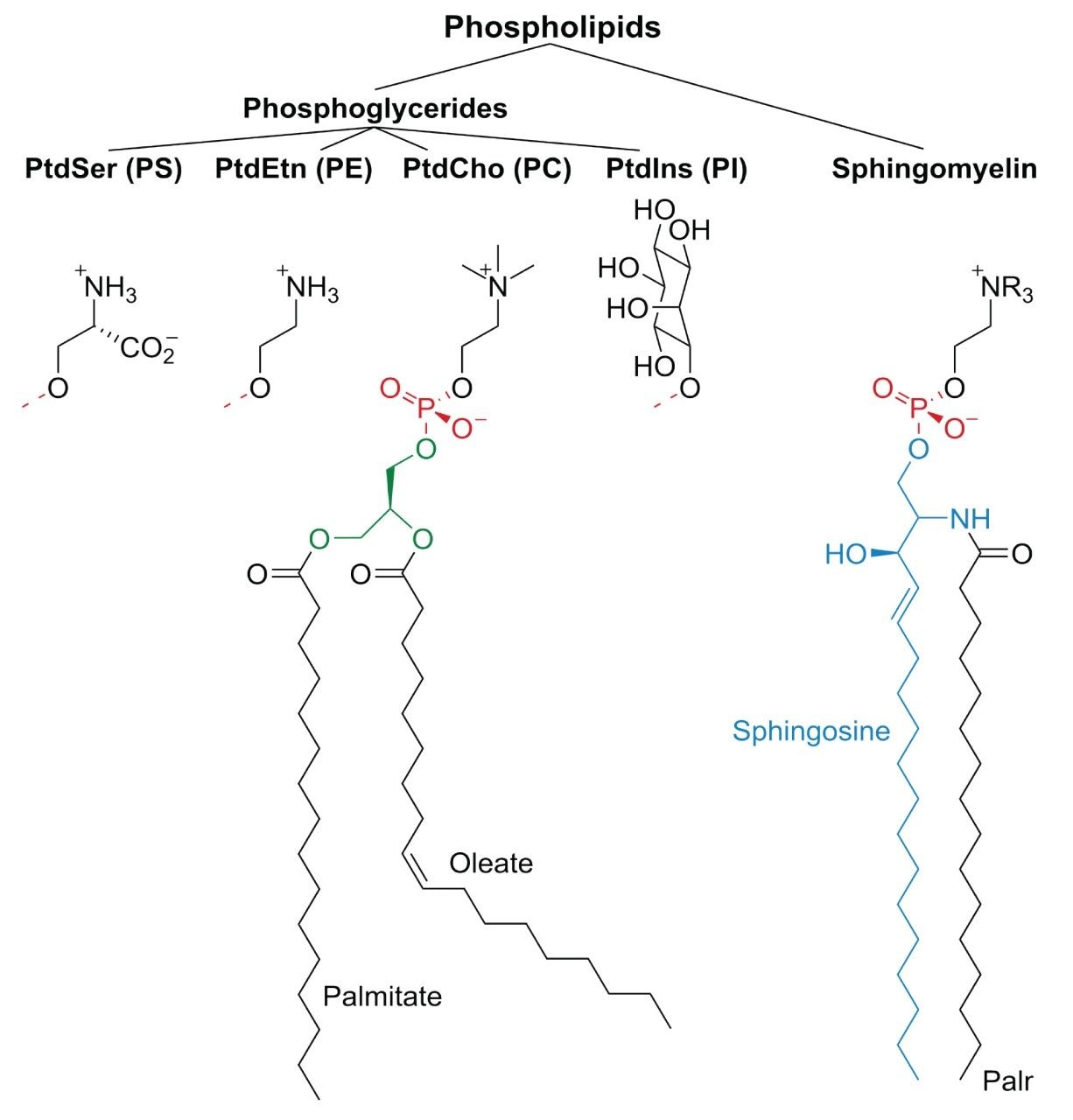
The present method of UltraPerformance Liquid Chromatography (UPLC) with UV detection offers advantages of high speed, resolution and simplicity for the separation and detection of phospholipids including phosphatidylcholine and phosphatidylethanolamine from rice and sunflower oil lecithin. The ultraviolet/visible spectrometer coupled with the UPLC system for the phospholipids detection has greater sensitivity over refractive index or flame-ionization detection. However, the UV detection restricts the use of common chromatographic solvents that are not transparent in 200 nm to 210 nm regions wherein phospholipids have tendency of absorbing the light energy. The Reverse Phase (RP) UPLC-UV system however can successfully engage solvents such as acetonitrile, ethanol, methanol, iso-propanol, and water. The significance of UV detection is that it has multiple choices of compositions of mobile phase to advance the isocratic or gradient elution. The available reverse phase methods are complex, laborious, and time-consuming, while this UPLC method is simple and short.
In this application note, we have developed a 15 minutes method for quantitative analysis of PE and PC on the ACQUITY UPLC H-Class Plus System with a PDA Detector.
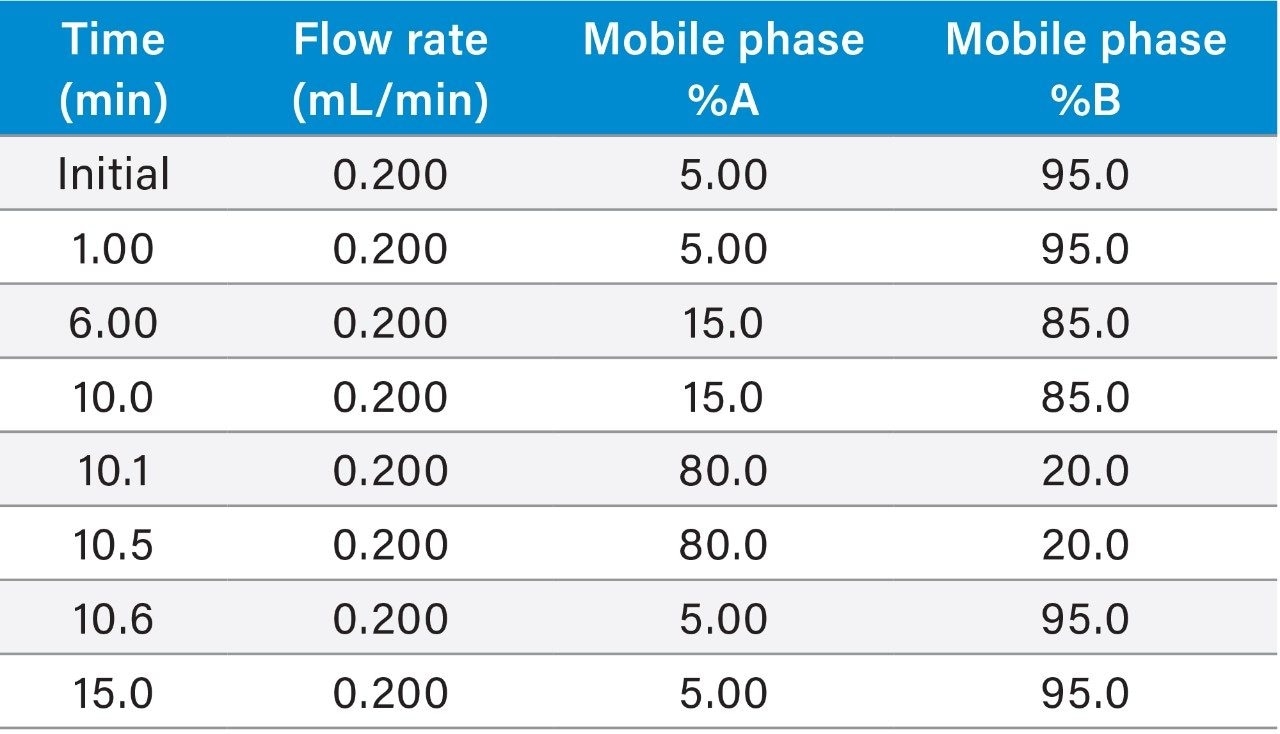
|
Instrument: |
ACQUITY UPLC H-Class Plus System with PDA Detector |
|
Column: |
ACQUITY UPLC BEH HILIC 1.7 μm, 100 mm × 2.1 mm |
|
Flow rate: |
0.2 mL/min |
|
Mobile phase A: |
10 mM ammonium formate and 0.05% ammonia solution in water |
|
Mobile phase B: |
Acetonitrile with 0.05% ammonia solution |
|
Column temp.: |
80 °C |
|
Sample temp.: |
15 °C |
|
Injection volume: |
10 μL |
|
Sample concentration: |
100 μg/mL for PE and 10 μg/mL for PC |
|
Wash and purge solvent: |
20:80 (Water:IPA) |
|
Seal wash: |
9:1 (water:methanol) |
|
Diluent: |
1:1 (chloroform:IPA) |
|
PDA fixed wavelength: |
205 nm |
|
Data acquisition rate: |
2 pts/sec |

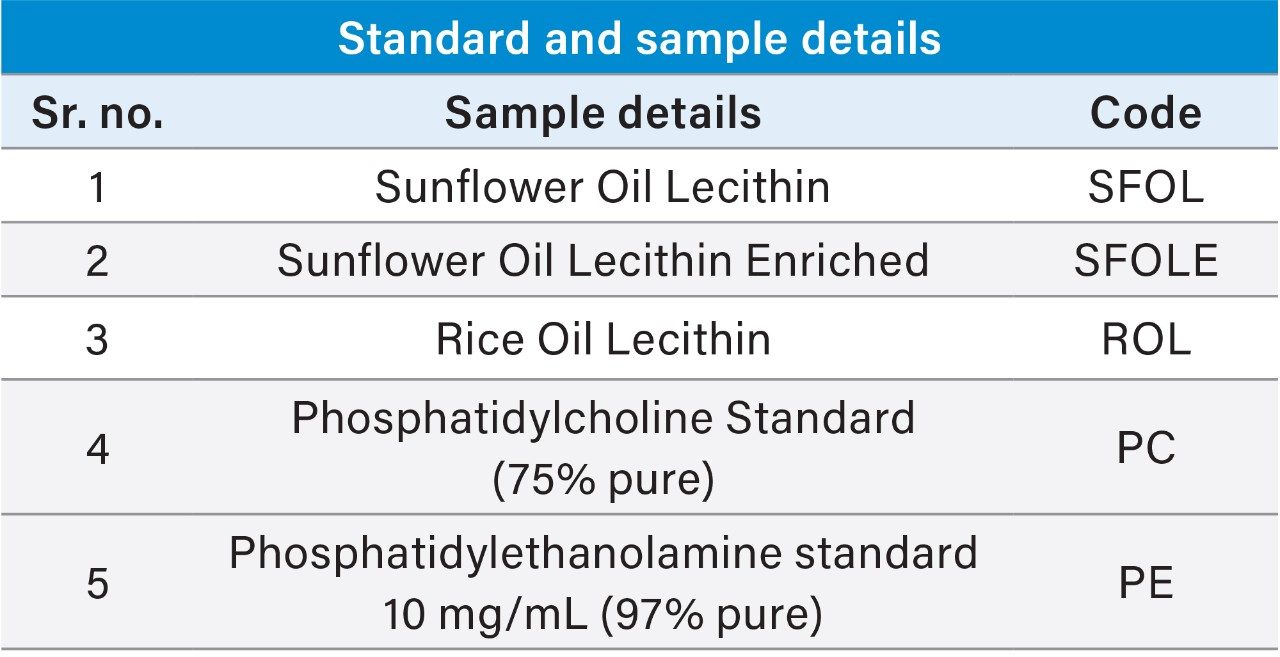
Accurately weighed 100 mg of PC standard (75% pure) and dissolved in 10 mL of diluent as a standard stock solution I of 10,000 µg/mL concentration.
Working standard was prepared by mixing 100 µL of standard stock solution I and 100 µL of PE standard labelled as 10 mg/mL (97% pure) and making up the volume to 1000 µL to make concentration 1000 µg/mL of each component mix.
Further dilutions for linearity were prepared by appropriate dilutions from the standard mix.
10,000 µg/mL stock solutions were prepared by weighing 100 mg of each sample and dissolved in 100 mL of diluent. Two separate concentrations were prepared by further diluting the stock solution to 100 µg/mL and 10 µg/mL for PE and PC analysis respectively.
Due to the huge difference in the concentration of PE and PC present in oil lecithin samples, separate sample dilutions were prepared for PE and PC. 1000 µg/mL stock solution of samples were diluted to 100 µg/mL (10 times) and 10 µg/mL (100 times) for PE and PC respectively and quantified against the calibration curve plotted from 1 ppm to 25 ppm.
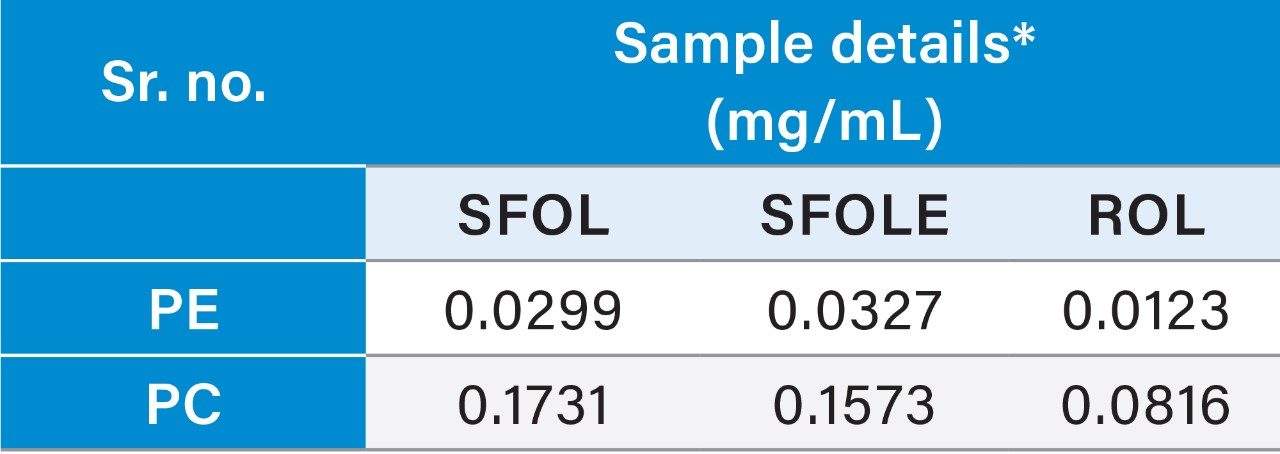
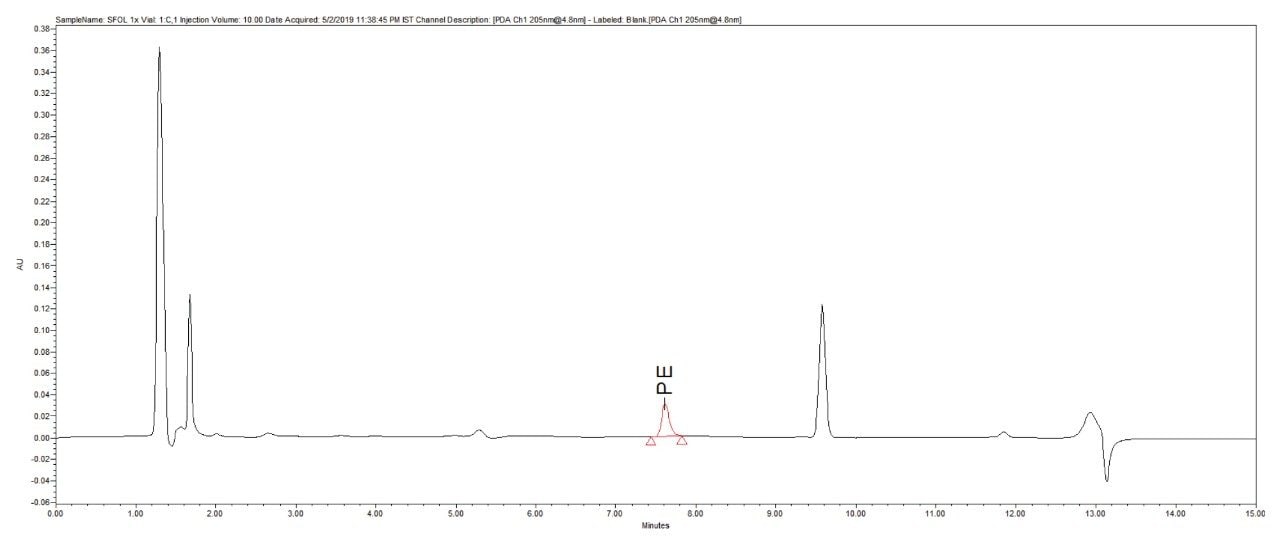
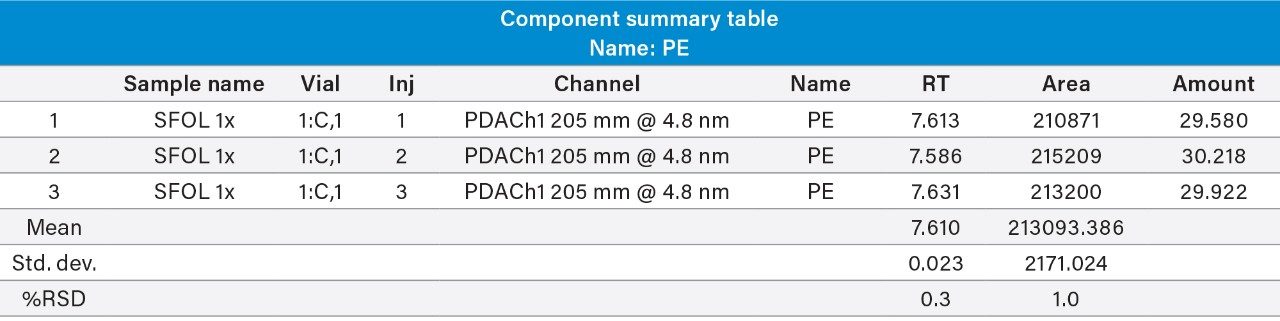
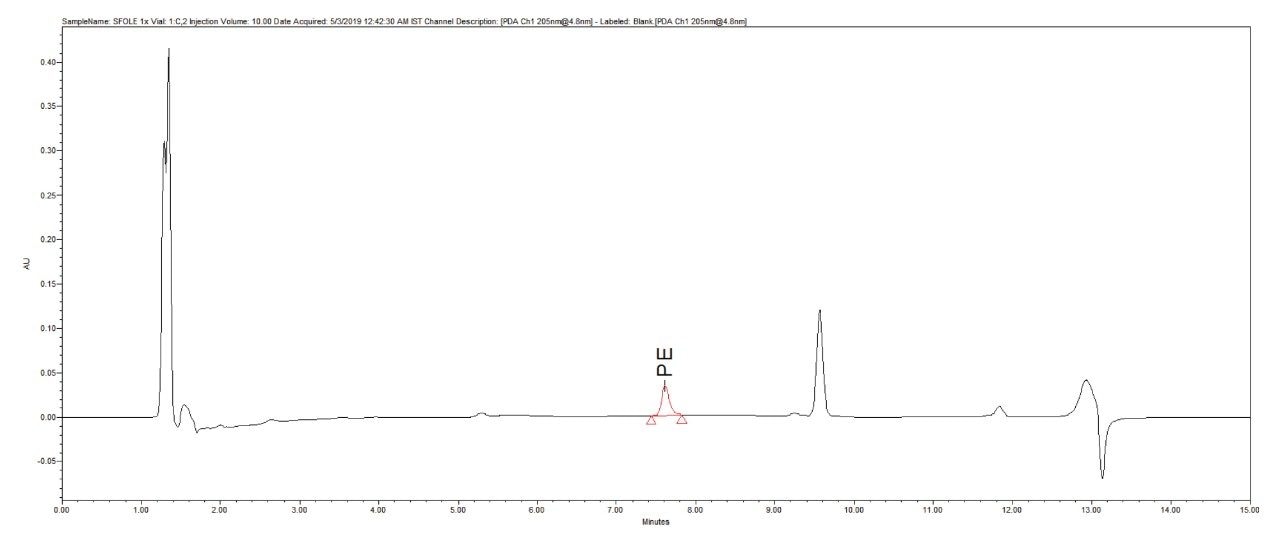
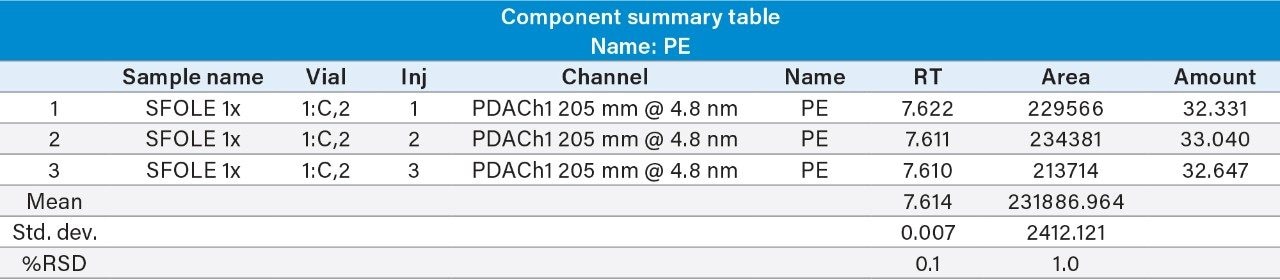
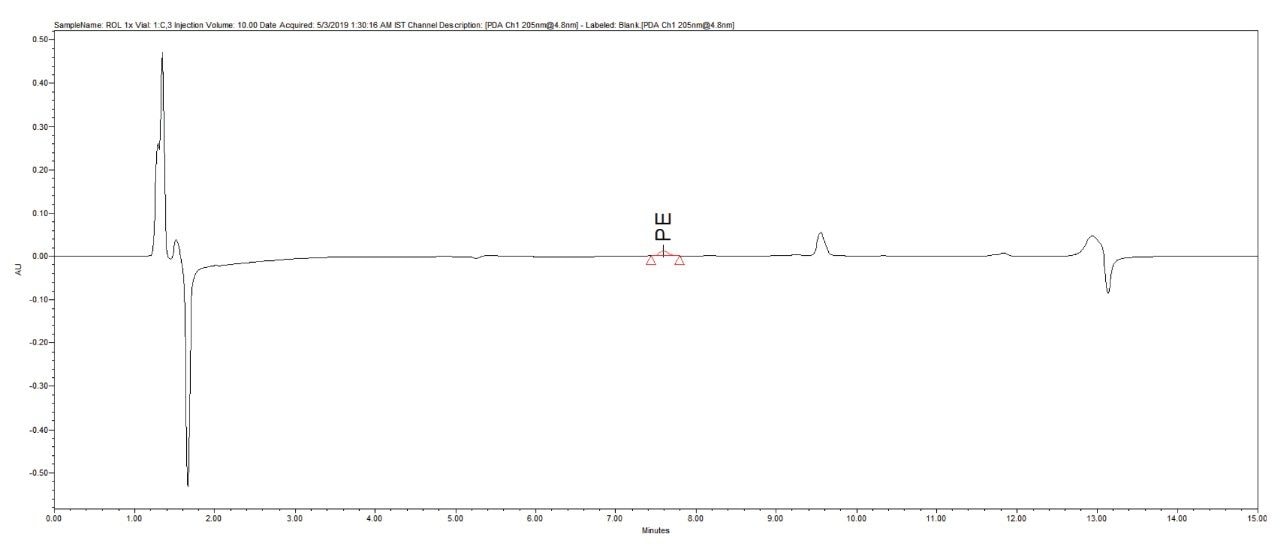
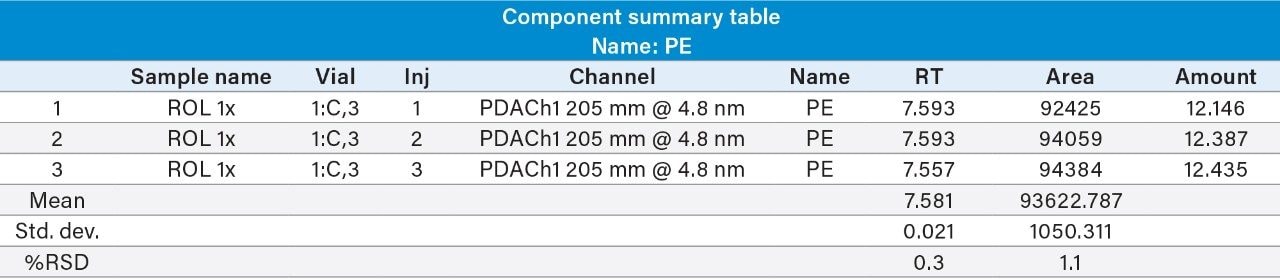
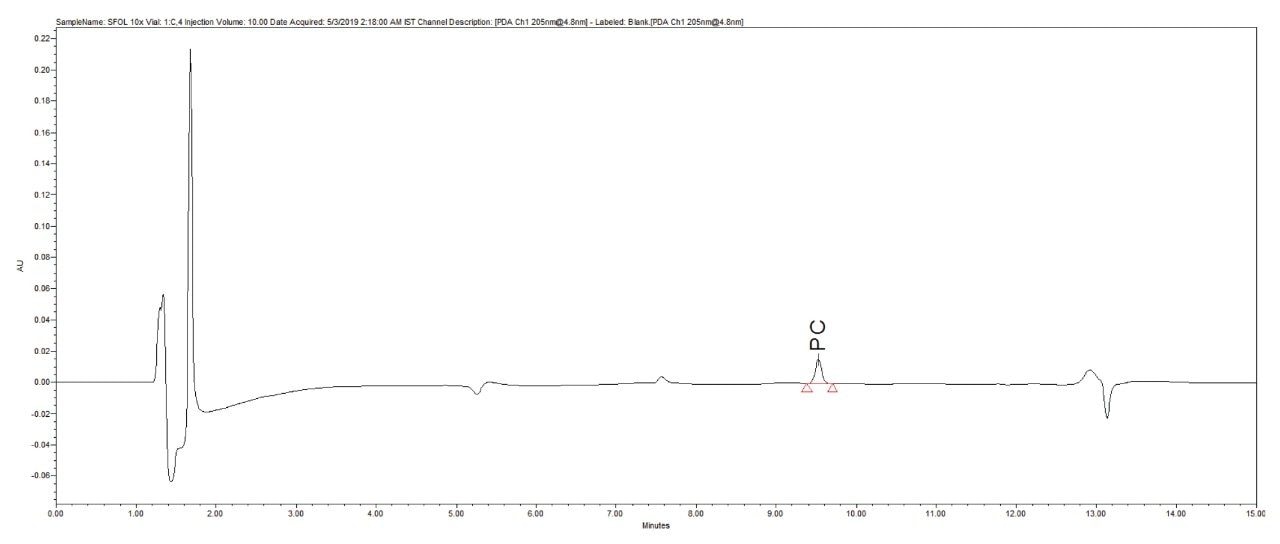
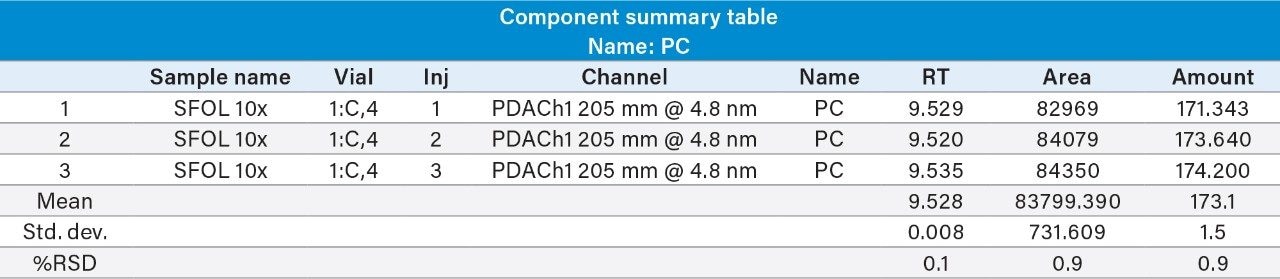
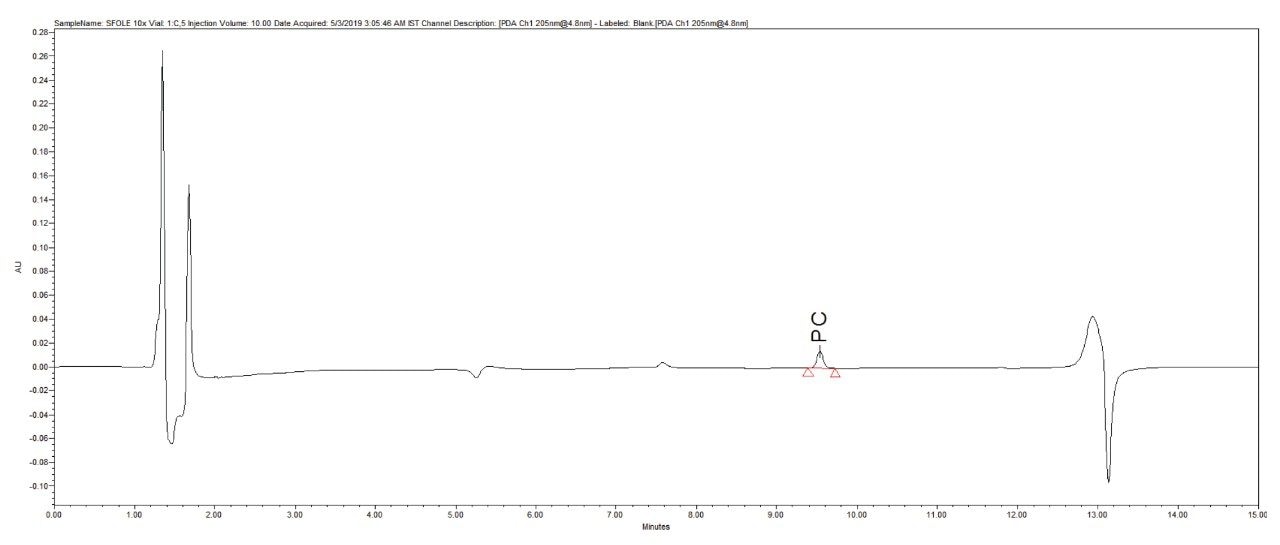
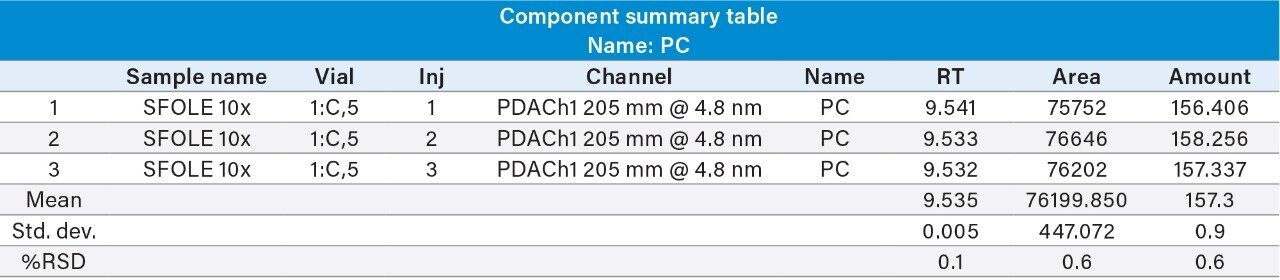
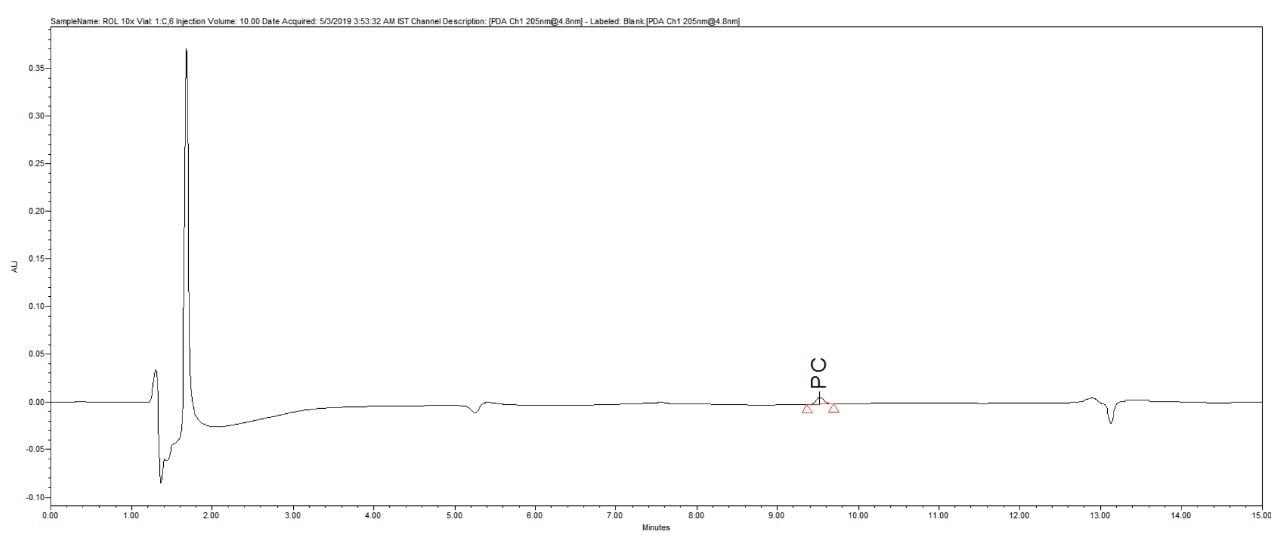
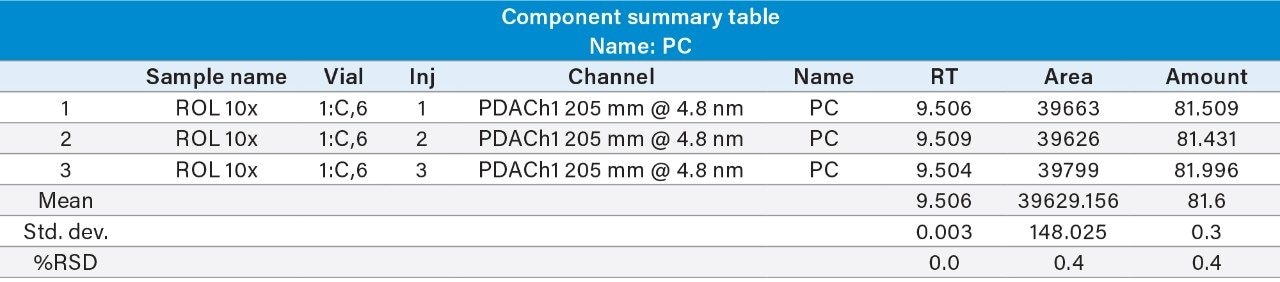
Reproducibility test was performed with 1 µg/mL (LOQ) concentration for PE and PC. %RSD of area observed in six replicate injections for both the components were within the limit.
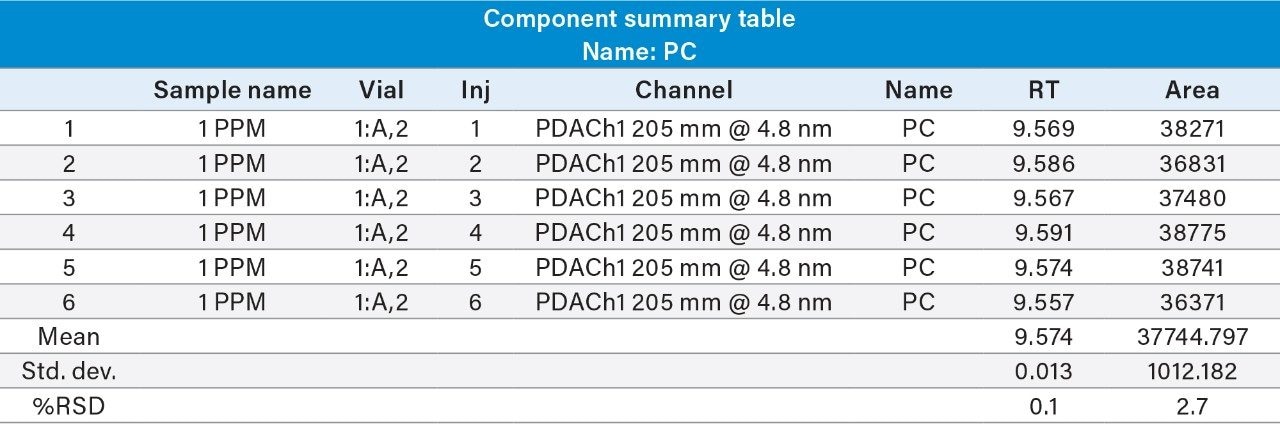
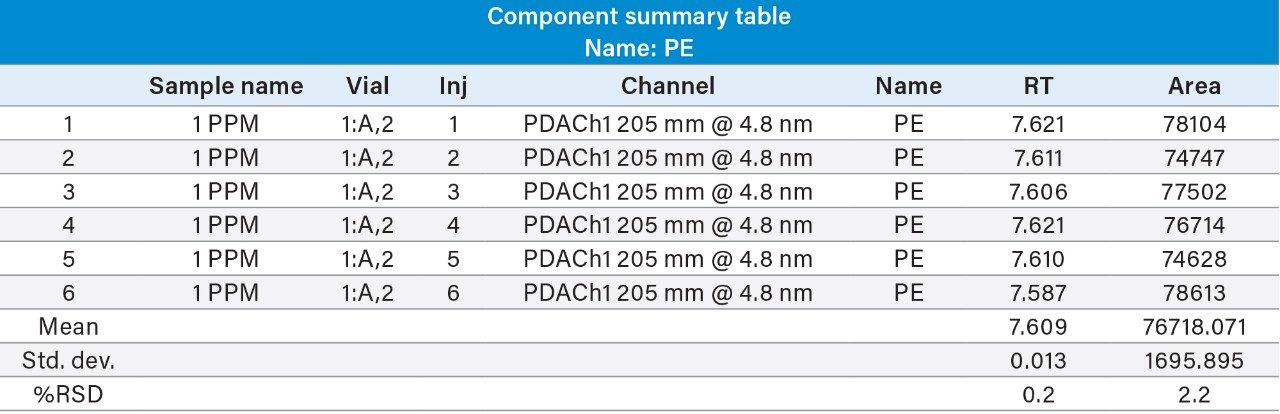
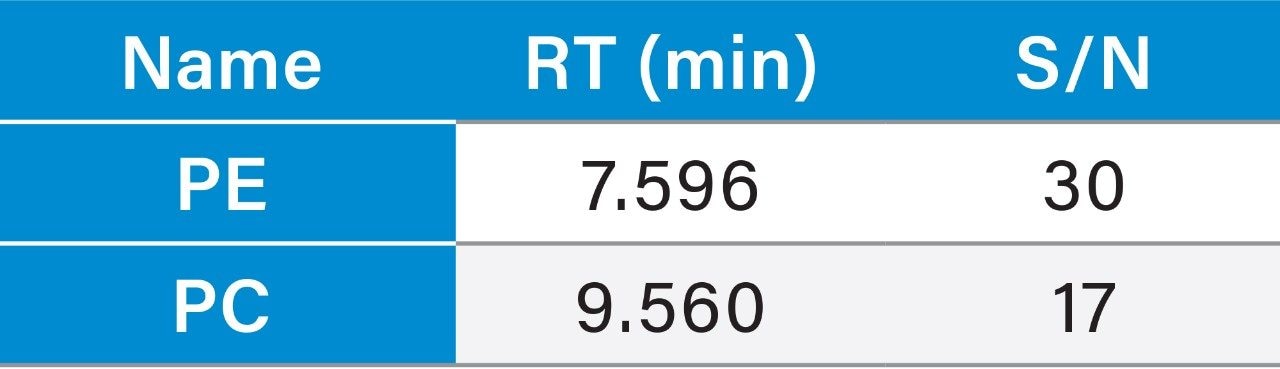
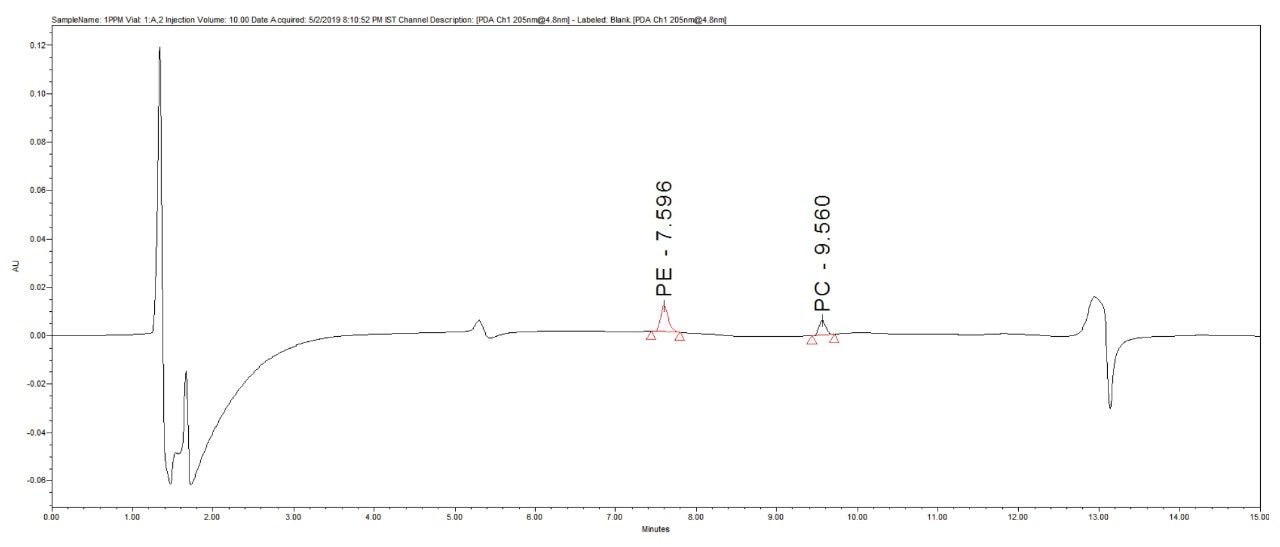
1 µg/mL (LOQ) concentration of the standard mix was injected and observed that the signal-to-noise ratio value is 30 for PE peak and 17 for PC peak.
Recovery study was performed for PE and PC by injecting 2, 10, and 15 µg/mL concentration of standard mix in neat solution and spiked 2, 10, and 15 µg/mL of PE and PC standard mix with final concentration of three samples and observed the recovery.

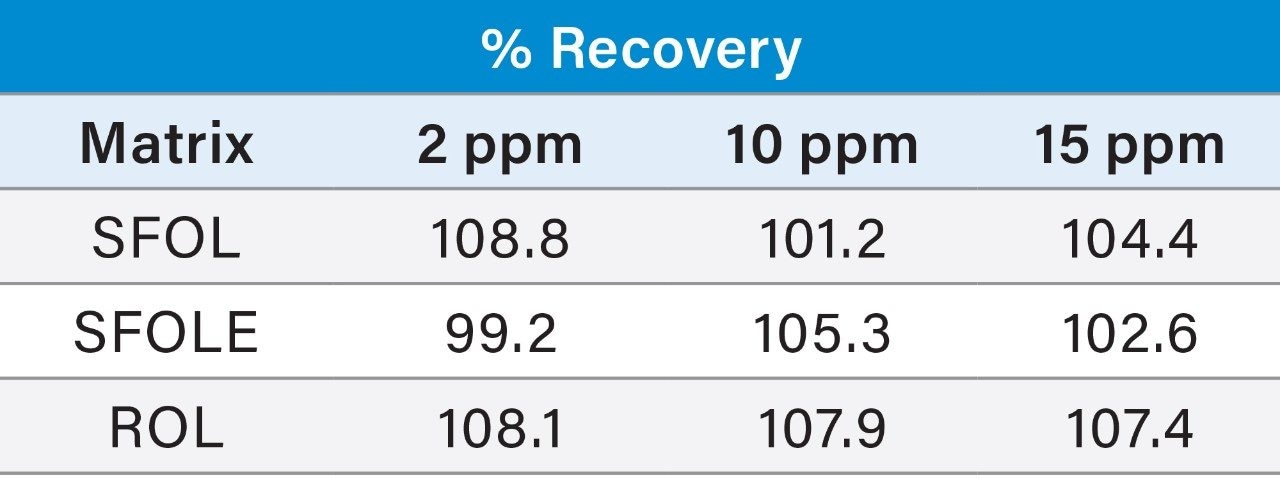
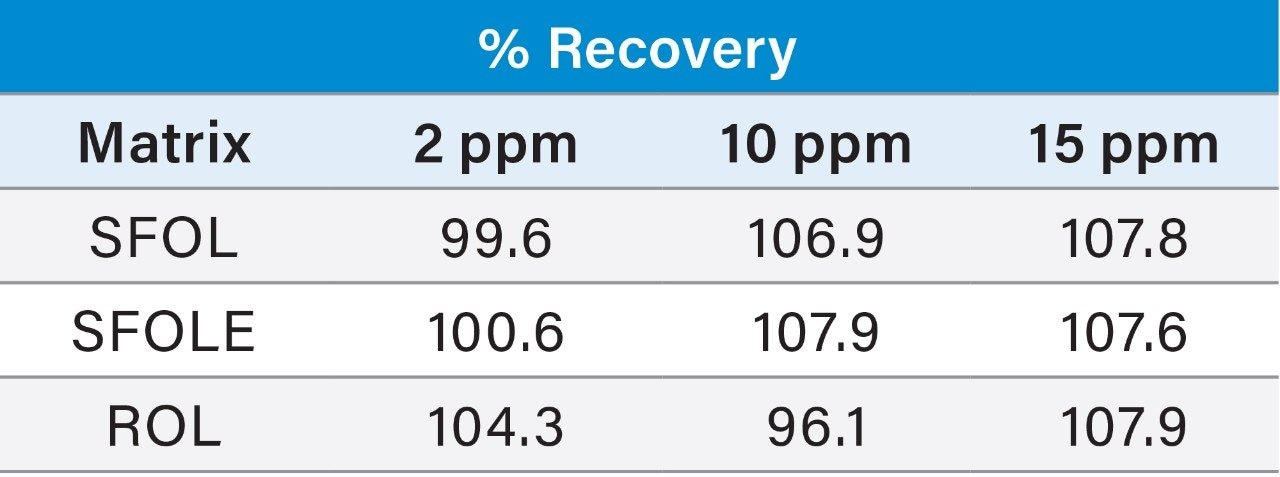
Prepared Linearity solutions of PE (97% pure) from 1 µg/mL, 2 µg/mL, 5 µg/mL, 10 µg/mL, 15 µg/mL, 20 µg/mL, and 25 µg/mL solutions and PC (75% pure) standard with different concentrations of 0.75 µg/mL, 1.5 µg/mL, 3.75 µg/mL, 7.5 µg/mL, 11.25 µg/mL, 15 µg/mL, and 18.75 µg/mL solutions and plotted calibration curve.
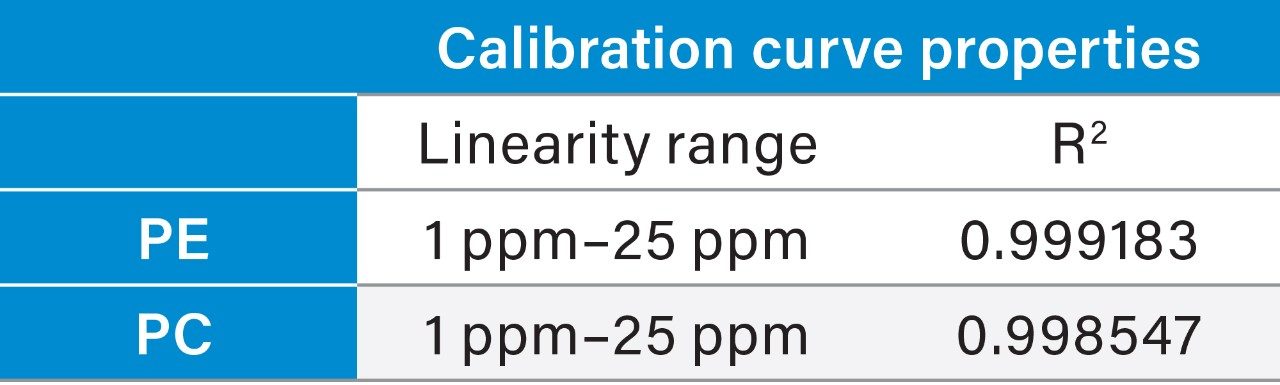
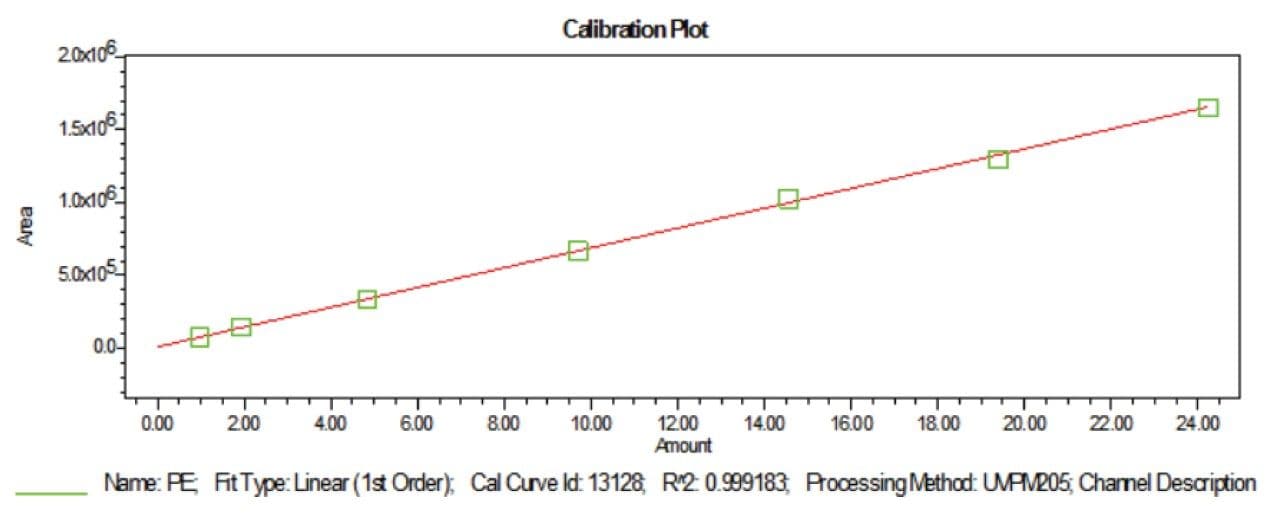
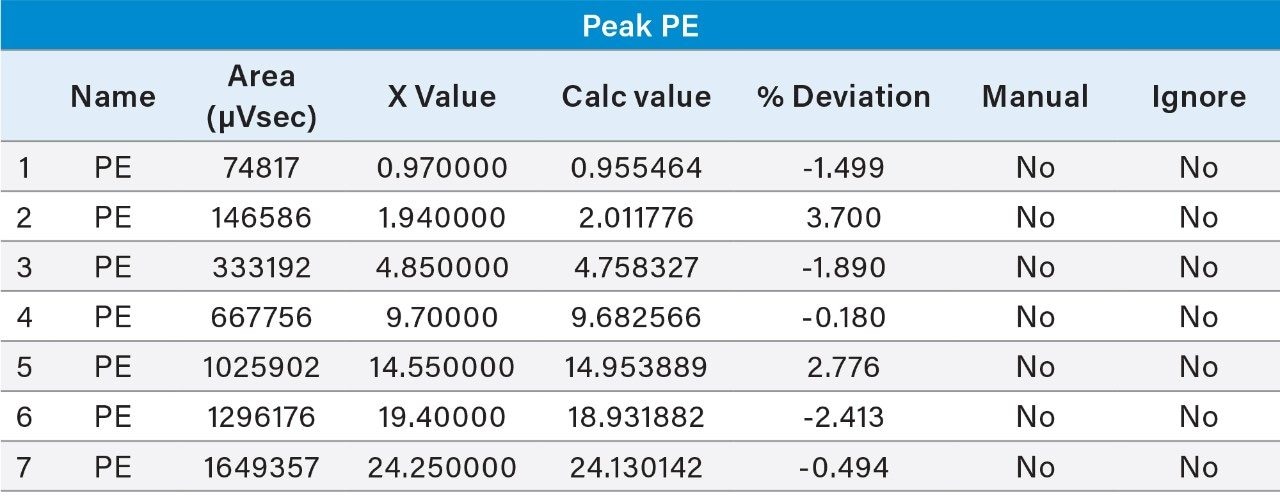
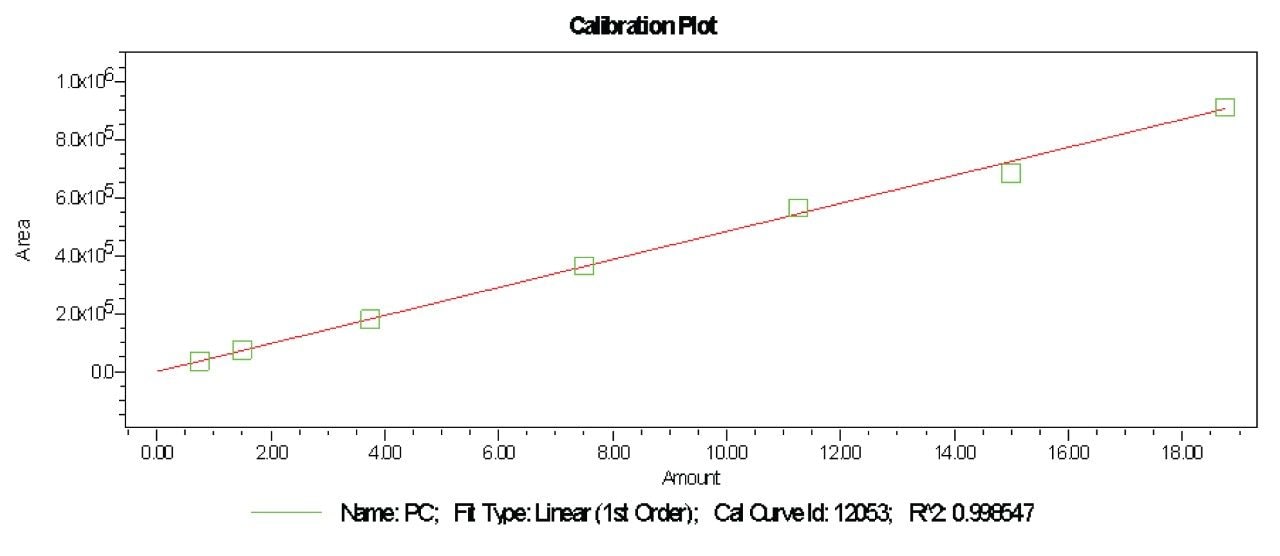
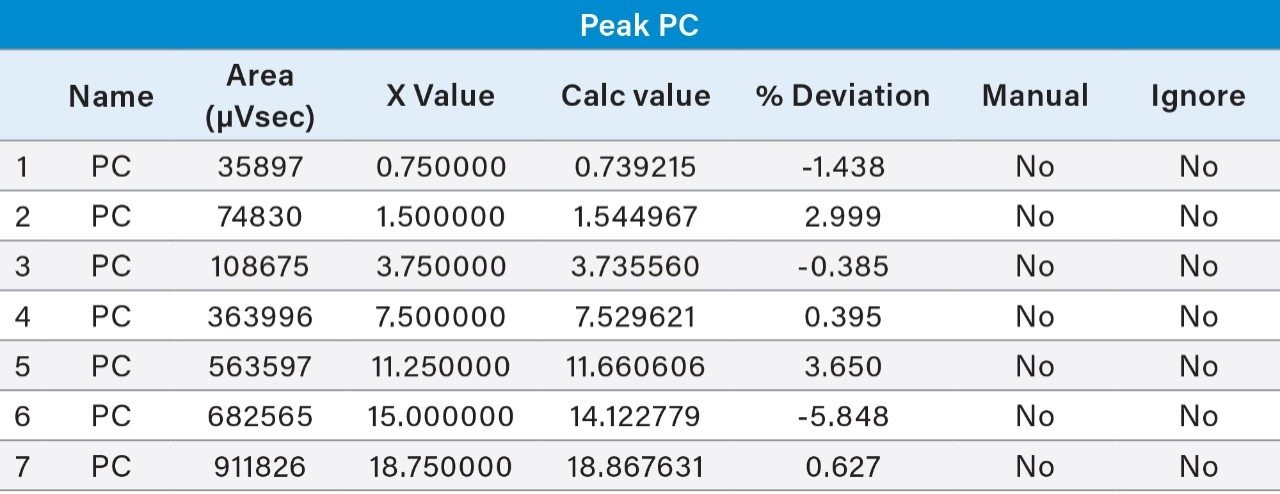
UPLC technology coupled with UV detection provides a unique solution for quantitative analysis of phosphatidylethanolamine and phosphatidylcholine from rice and sunflower oil lecithin.
Authors wish to thank Mr. Geemon Korah, Director, CEO, and Mr. Sasheendran Vijayan, Head-Innovations, Kancor Ingredients Ltd. for their immense support for conducting the research collaboratively.
720006715, December 2019