
This application note describes the development and validation of a comprehensive screening method based on UPLC-MS/MS for the detection of over 150 veterinary drugs, from many different classes, in animal muscle tissue. Consolidation of many compounds into one screening method improves operational efficiency and reduces costs. Muscle tissues were extracted using a generic liquid extraction using oxalic acid in acetonitrile followed by a rapid and cost-effective clean-up using dispersive solid-phase extraction (dSPE) and determination by UPLC-MS/MS using electrospray and polarity switching. Despite using generic conditions, a UPLC system can provide increases in sample throughput by speeding up analysis times, maintains good peak shape across a wide range of different compounds, ensures sufficient retention of polar compounds, and the separation of critical pairs. The method was successfully validated in muscle tissue using the guideline document on screening methods that supplements Commission Decision 2002/657/EC. In all cases, values for CCβ were established at concentrations below MRLs and in most cases at 0.1 or 1.0 µg/kg. As validation criteria have been met, the method is considered sensitive, robust, specific, and fit for the purpose of screening for veterinary drug residues.
Many countries have an integrated approach to food safety that aims to assure a high level of food safety, animal health, animal welfare, and plant health through coherent farm-to-table measures and adequate monitoring, while ensuring the effective functioning of trade. Most countries have regulatory approval systems in place which establish which veterinary drug treatments are permitted and set Maximum Residue Limits (MRLs). An MRL is the maximum concentration of residue resulting from the use of a veterinary medicinal product which may be accepted to be legally permitted or recognized as acceptable in or on a food. The use of non-approved medicines is prohibited. Prohibited veterinary medicines have no MRLs. Minimum Required Performance Limits (MRPLs) were established by Commission Decision 2002/657/EC,1 but only set for a limited number of banned substances, the European Union Reference Laboratories, set Recommended Concentration (RC) values to improve and harmonize the performance of analytical methods used for those substances for which MRLs have not been established.2
Monitoring of veterinary drug residues is required to support regulatory frameworks by checking compliance with MRLs and the other action limits, but also by industry to protect the customer and the industry/brand. Within Europe there is a very low non-compliance rate (0.3% of samples test “positive”)3 and this drives a two-stage approach. Screening methods are initially used to detect the presence of a substance or class of substances at the level of interest. Methods, such as those based on microbial growth inhibition, are used to sift large numbers of samples for potential non-compliant results in a cost-effective manner. Such test kits often give false positive results if used outside their specific scope, both in terms analyte coverage and sample type. Any “suspect positive” result from a screening test triggers the second stage; repeat analysis for confirmation. The sample must be re-analyzed to quantify the residue and meet acceptance criteria for identification.
LC-MS/MS can extend the scope of the method to many analytes from different classes, applicable for multiple matrices. This approach for screening purposes is becoming popular as it can provide good specificity, sensitivity, and a low rate of false-positive samples. Most screening workflows focus on a targeted approach, looking for known substances at a pre-determined cut off level to evaluate if the sample does or does not contain veterinary drug residue at a concentration above the Screening Target Concentration (STC). The STC is defined as the lowest concentration for which it has been demonstrated that a compound can be detected in at least 95% of samples (<5% of false compliant results).4 The further the STC is below the regulatory/action limit, the lower the probability of obtaining a false compliant result in samples containing the drug at that limit. The same methodology can be used subsequently for confirmation (repeat analysis) if validated for that purpose, but the relevant analytical quality control acceptance criteria need to be met.
This application note describes the validation of a multiresidue method for the determination of residues of veterinary drugs from over 13 different classes in muscle tissue from various species, using the ACQUITY UPLC I-Class PLUS coupled to the Xevo TQ-XS.
Samples of minced muscle tissue, from various animal species, were purchased from local supermarkets.
Muscle tissues were extracted using a generic liquid extraction using oxalic acid in acetonitrile followed by a dispersive solid-phase extraction (dSPE) clean-up (see Figure 1 for more detail). Extracts were stored at -20 °C and were analyzed by LC-MS/MS within 2 days of extraction due to concerns over stability of some analytes.
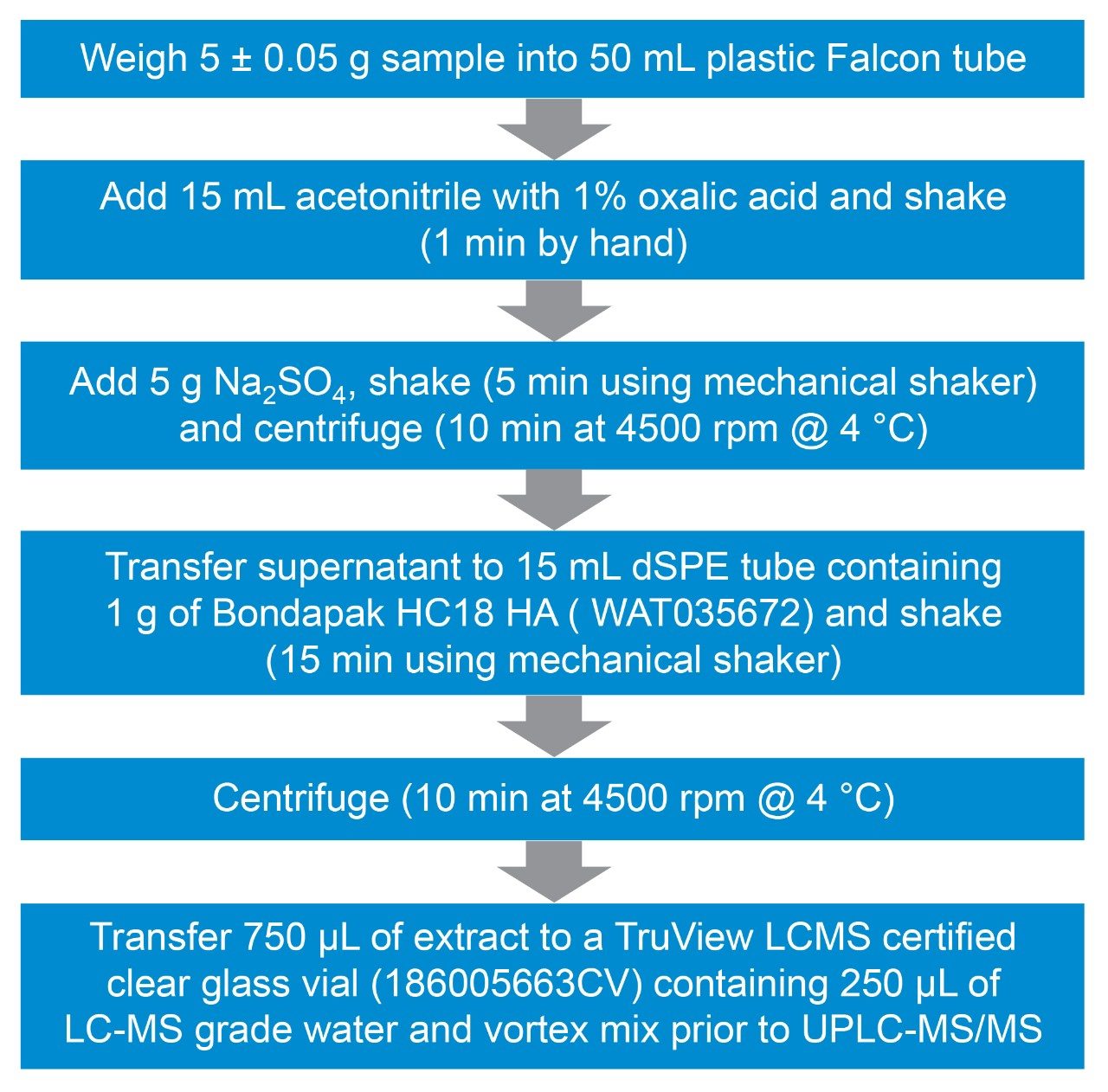
Matrix-matched standards were prepared in bovine muscle tissue extract, previously shown to be blank, at concentrations between 0.05 and 20.0 µg/kg.
|
LC Conditions |
|
|---|---|
|
LC system: |
ACQUITY UPLC I-Class PLUS with FL Sample Manager |
|
Column(s): |
HSS T3, 1.8 µm, 2.1 x 100 mm (186003539) with ACQUITY Column In-Line Filter (205000343) |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
1 µL (Full Loop) |
|
Strong wash solvent: |
0.1% formic acid in acetonitrile/methanol (1:1, v/v) |
|
Weak wash solvent: |
0.1% formic acid with 0.1 mM ammonium formate (aq) |
|
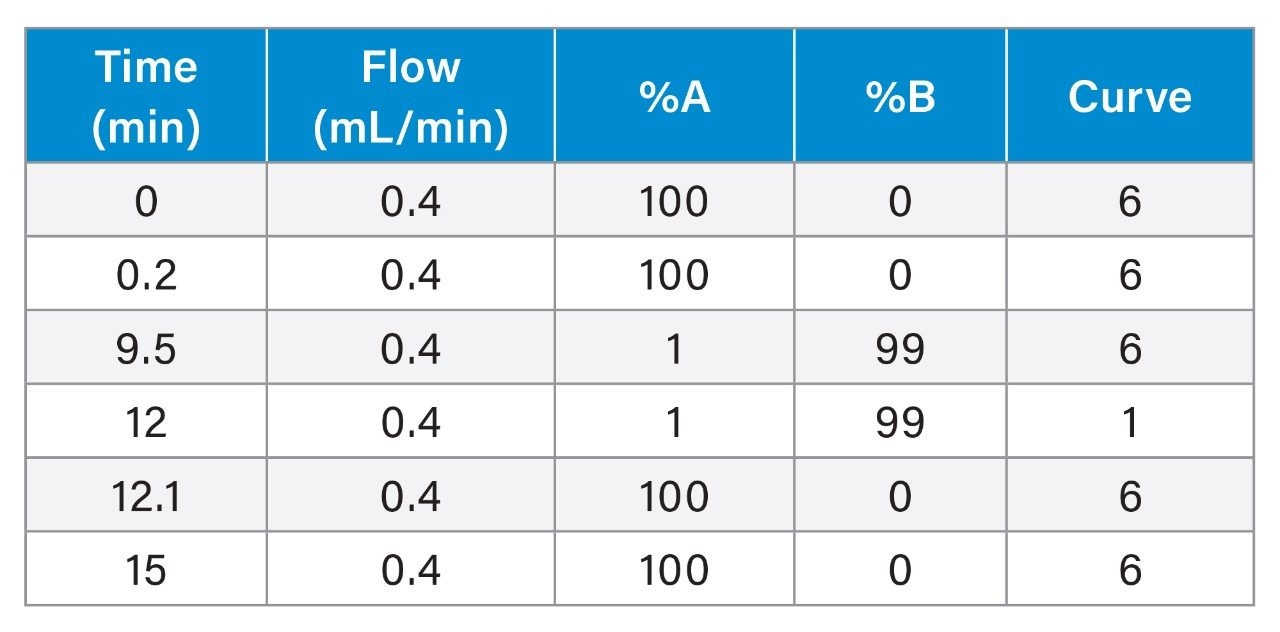
Mobile phase A: |
0.1% formic acid with 0.1 mM ammonium formate (aq) |
|
Mobile phase B: |
0.1% formic acid in acetonitrile/methanol (1:1, v/v) |
|
MS system: |
Xevo TQ-XS |
|
Ionization mode: |
Electrospray using polarity switching |
|
Capillary voltage (kV): |
ESI+ 1.0, ESI- 2.5 |
|
Source temperature (°C): |
150 |
|
Desolvation temperature (°C): |
600 |
|
Desolvation gas flow (L/Hr): |
1000 |
|
Cone gas flow (L/Hr): |
150 |
|
MS software: |
MassLynx v4.2 |
|
Informatics: |
TargetLynx XS Application Manager |
Multiple MRM transitions per compound were acquired. The dwell times were set automatically using the autodwell function to give a minimum of 10 data points across each peak. The transitions used in this application can be downloaded from Waters Marketplace from the Quanpedia, MassLynx section.
The protocol from the guideline document4 that supplements Commission Decision 2002/657/EC regarding the validation of screening methods to assess the performance of the method was used to evaluate method performance. The principle of this validation was to evaluate the range of analytical responses in un-spiked versus spiked samples and to set a cut off level ensuring that the lowest response for the spiked samples does not overlap with the highest response for the un-spiked samples. The cut off level is the response from a screening test which indicates that a sample contains an analyte at or above the STC. Screening methods do not have to fulfil requirements of Commission Decision 2002/657/EC with respect to repeatability, reproducibility or trueness. Validation was carried out through analysis of 21 blank muscle samples (bovine, porcine, and poultry) and replicates of those same samples spiked at three different concentrations (0.1, 1.0, and 10 µg/kg) to establish the STC. In the case of drugs with complex MRL definitions, the parent and/or metabolites have been sought as marker residues, but no attempt was made to include any chemical conversion step to a single marker residue (such as hydrolysis or oxidation) as this would adversely affect performance for other compounds.
Consolidation of the many compounds previously covered in class- or compound-specific methods improves operational efficiency and reduces costs. As such this approach must cope with many compounds with varying chemical characteristics, it relies on generic extraction conditions and limited clean-up to ensure adequate analyte recovery. Some classes, such as aminoglycosides, are too polar to be included in the scope of this multiresidue method and rely on separate class-specific methods.5
The mixture of acetonitrile and water can extract a wide range of analytes from the matrix because it provides adequate extraction efficiency for both polar analytes as well as nonpolar compounds. Tetracyclines chelate with multivalent cations and proteins in sample resulting in low extraction efficiencies. 1% oxalic acid was added to extraction solvent to minimize analyte chelation with metal ions present in food matrices to aid recovery of tetracyclines. Due to the wide range in analyte chemical properties any clean-up must be generic and non-selective. The objective was to remove some of the co-extractives that impact the performance without significant loss of analytes. Phospholipids, other lipids, and pigments can cause matrix effects (typically ion suppression) as well as contaminate columns and LC-MS/MS hardware increasing the frequency of replacement of columns and maintenance of systems. When left unchecked, this often leads to a rapid and significant decline in performance resulting in failed batches and loss of confidence in robustness of LC-MS/MS system. A simple and cost-effective dSPE clean-up was used, which removed much of the co-extractives, including 40% of phospholipids monitored, whilst avoiding significant loss of analytes.
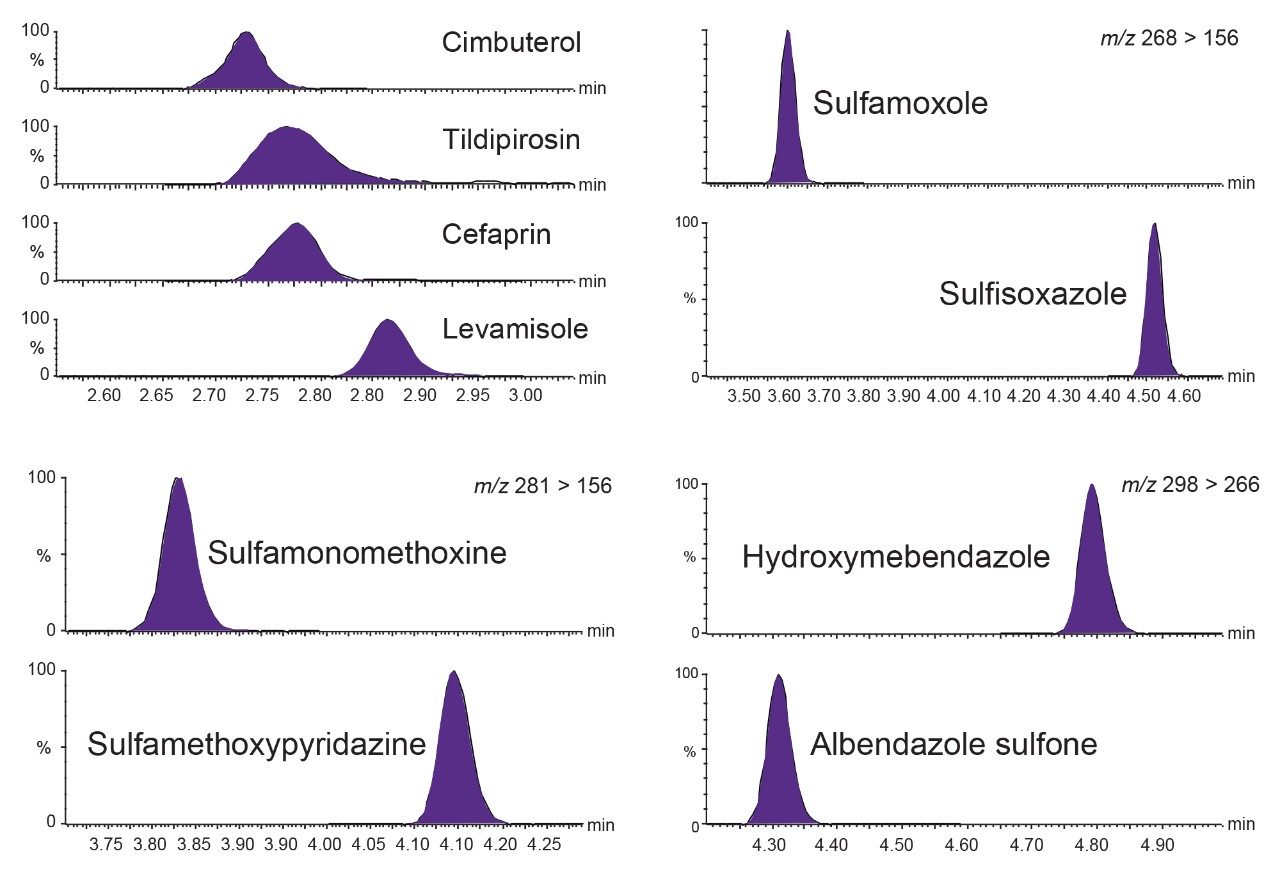
The HSS T3 Column provided excellent retention and peak shape for all the analytes and separation of isobaric compounds (Figure 2). All peaks eluted between 1.65 and 11 minutes with a total run time of 15 minutes.
Detection capability (CCβ) is the smallest amount of a substance that may be detected in a sample with a false negative rate of 5%. For authorized pharmacologically active substances the CCβ shall be less than the MRL but for prohibited or unauthorized substances, the CCβ shall be as low as analytically achievable. The CCβ was evaluated by a comparison of the Threshold value (T) and Cut-off factor (Fm).
The Threshold value (T) is a value corresponding to the minimum analytical response above which the sample will be truly considered as positive. This parameter was determined by analyses of 21 blank samples from different origins and calculated using the equation:
T = B+(1.64×SDb),
which considers the mean value of the blank/noise “B” and the standard deviation of the blank/noise “SDb”. The 1.64 factor is the one-tailed Student t-value for infinite degrees of freedom at a significance level, β = 0.05. The Cut-off factor (Fm) was determined by analyses of 21 blank samples spiked at the level of interest for each analyte. The response was determined for each analyte and the Fm calculated using the following equation:
Fm = M–(1.64×SD),
which considers the mean response “M” and the standard deviation “SD” for each analyte.
According to Commission Decision 2002/657/EC, CCβ is validated when Fm>B. The rate of false positives is acceptable (i.e. 5%) when Fm>T. So, when B<T<Fm, the CCβ is truly below the spiked concentration, the STC being tested, indicating the false negative rate = 5% and the false positive rate <5%. When T>Fm, more than 5% of the spiked samples will be considered as negative so the CCβ cannot be established at that spiking concentration so the assessment is repeated at higher concentrations.
Figure 3 shows Blank (B), Threshold value (T), and Cut-off factor (Fm) for dapsone, a banned substance, in mixed muscle samples. Using the spikes at 0.1 µg/kg, B<T<Fm, so the STC can be established at 0.1 µg/kg, well below the RC of 5 µg/kg for muscle.
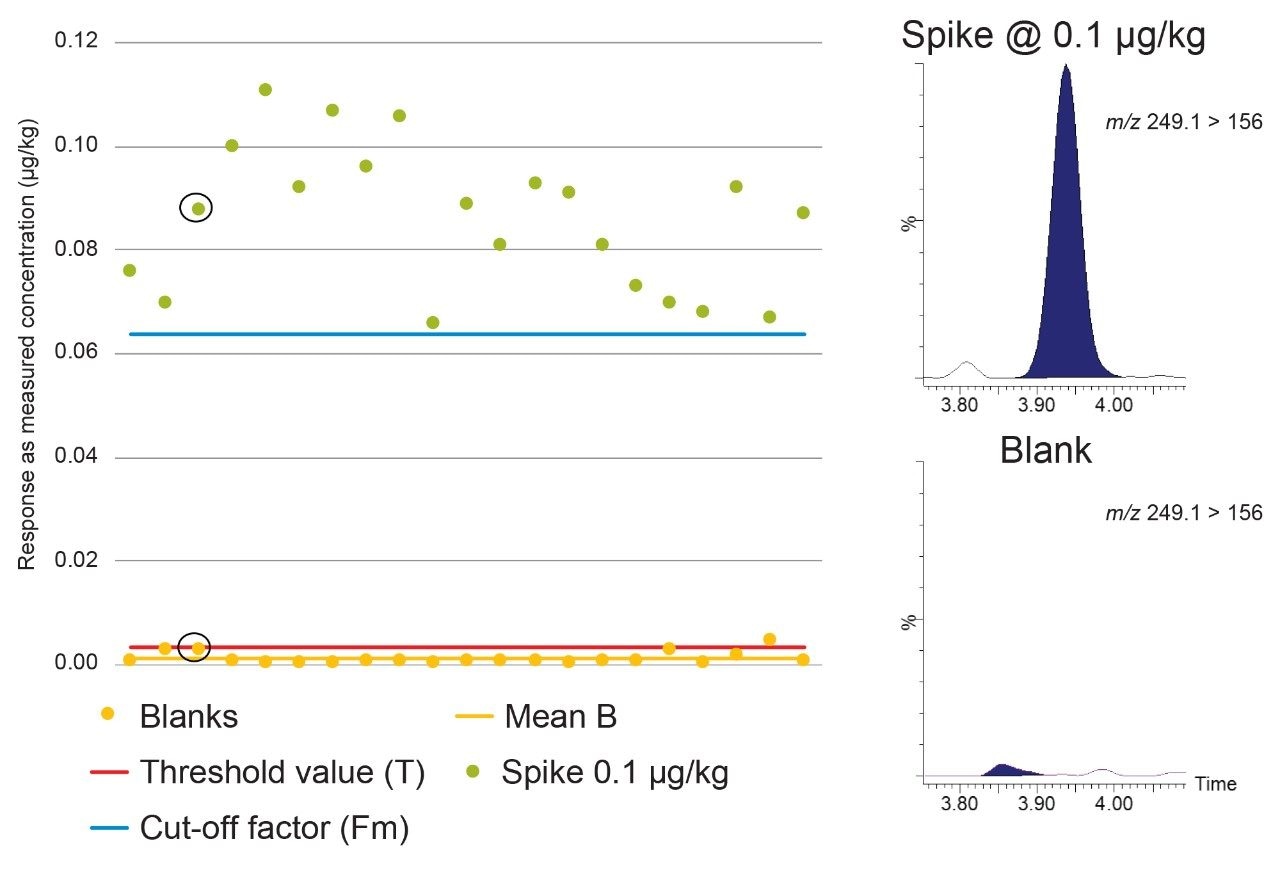
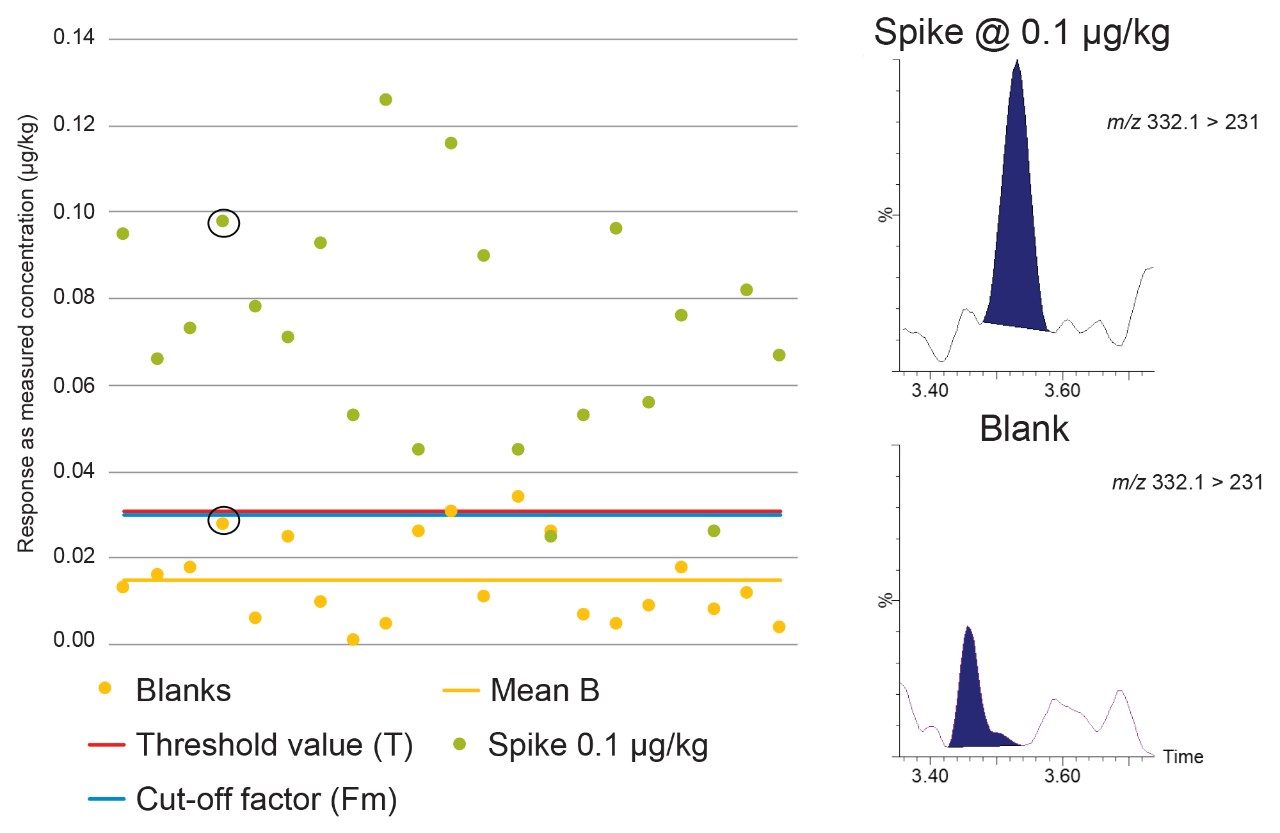
Figure 4 shows Blank (B), Threshold value (T), and Cut-off factor (Fm) for albendazole sulfoxide in mixed muscle samples. Using the spikes at 0.1 µg/kg, B<T<Fm, so the STC can also be established at 0.1 µg/kg, well below the MRL of 100 µg/kg for bovine muscle (sum of albendazole SOX, albendazole SON, and albendazole 2-aminosulphone).
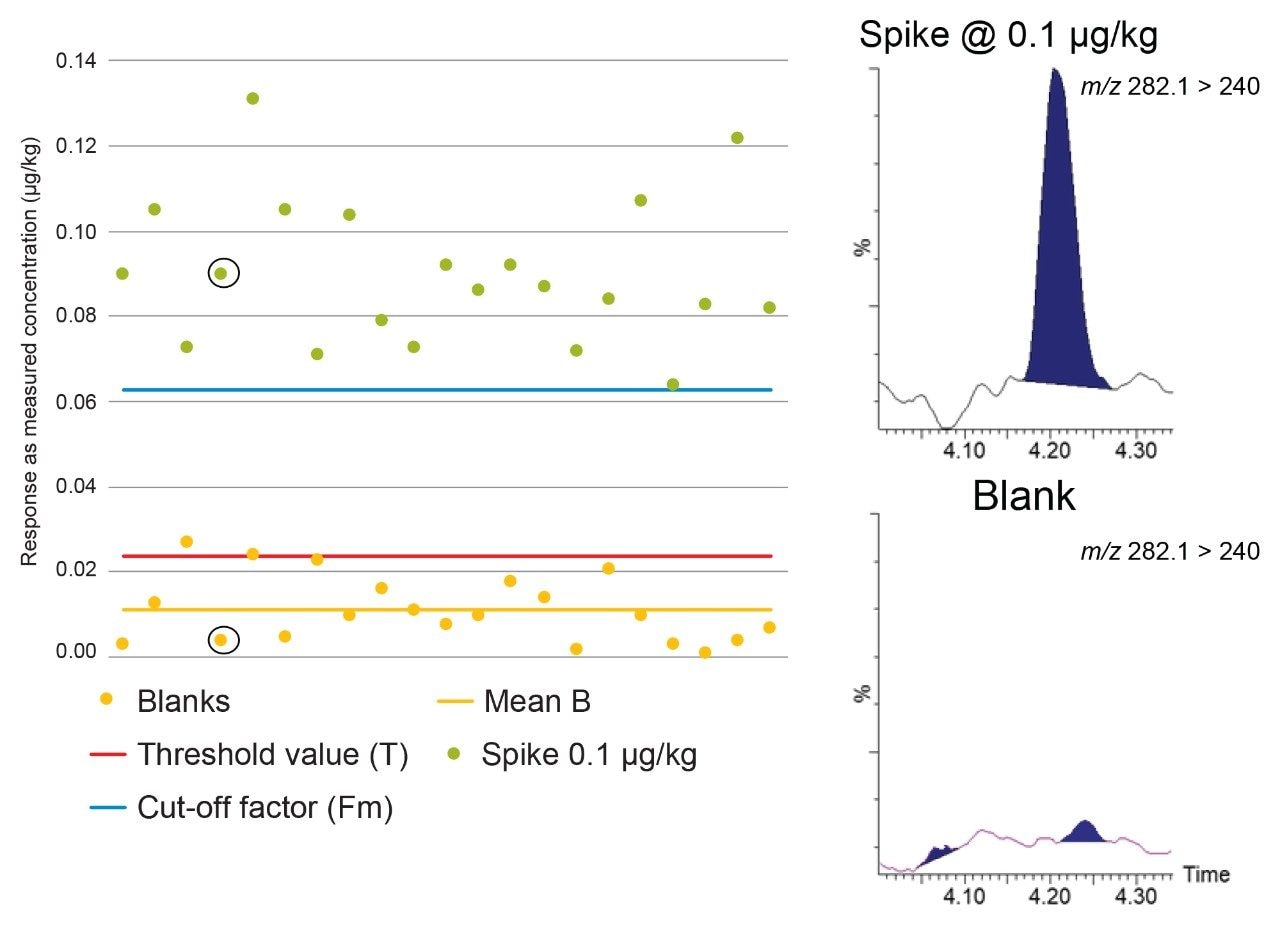
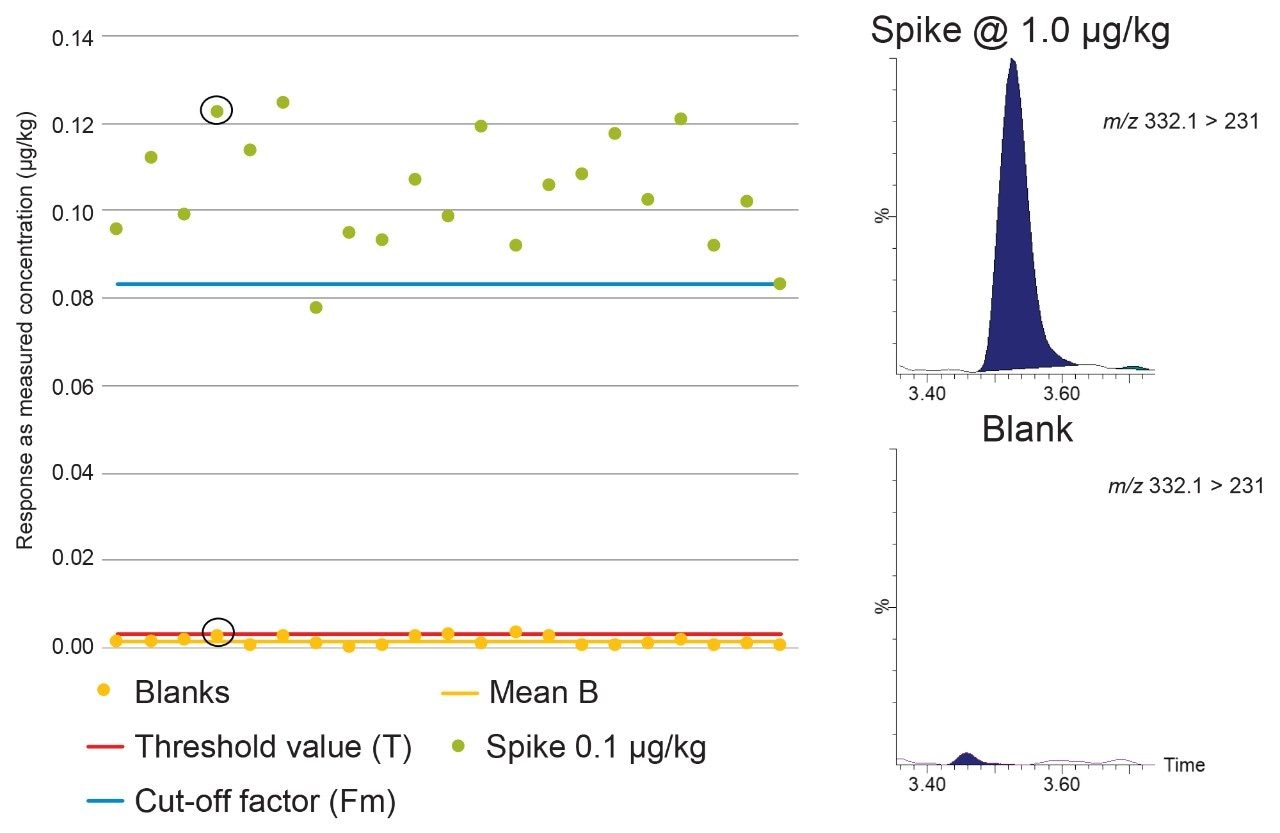
In a third example, Figure 5 shows Blank (B), Threshold value (T), and Cut-off factor (Fm) for ciprofloxacin in mixed muscle samples. Using the spikes at 0.1 µg/kg, T>Fm, so the STC cannot be established at 0.1 µg/kg. In contrast Figure 6 shows the data using the spikes at 1.0 µg/kg where B<T<Fm, so CCβ was established ≤1.0 µg/kg, well below the MRL of 100 µg/kg for bovine muscle (sum of enrofloxacin and ciprofloxacin).
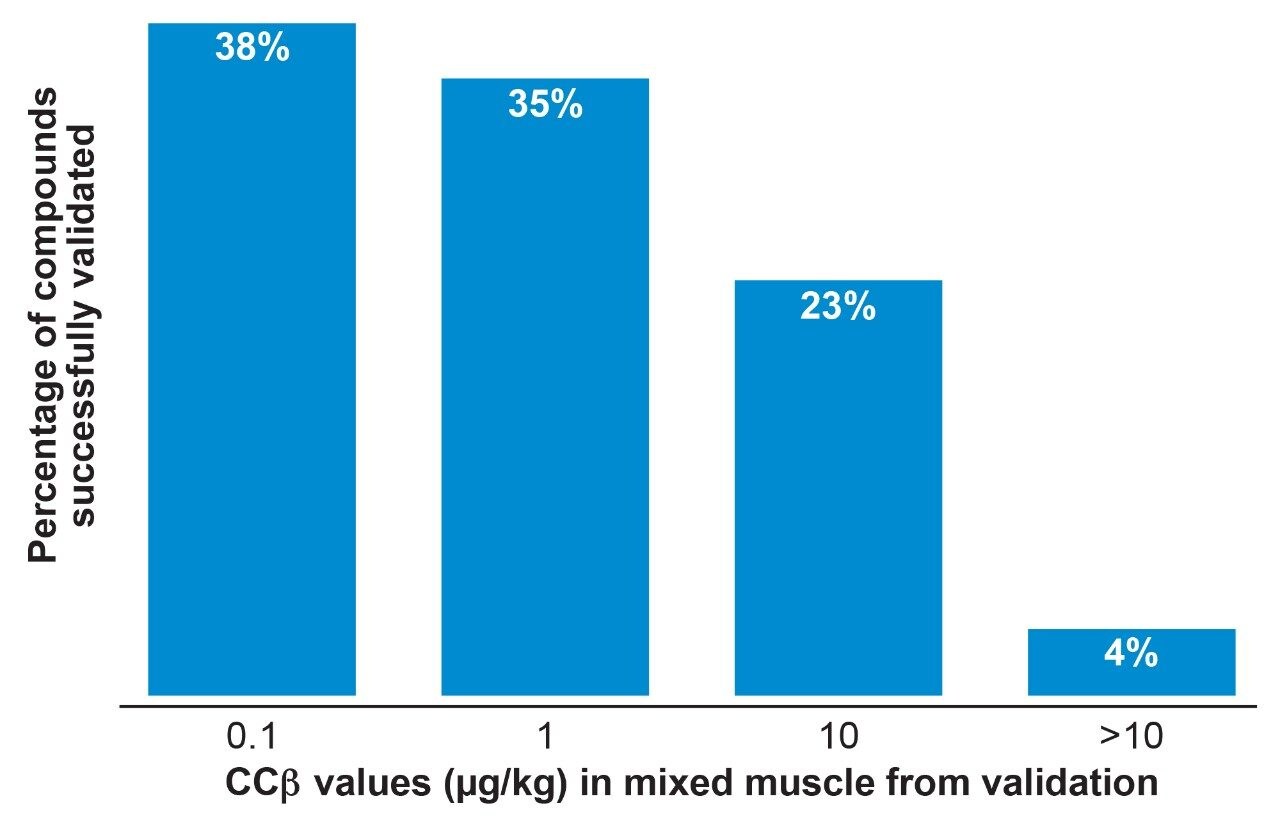
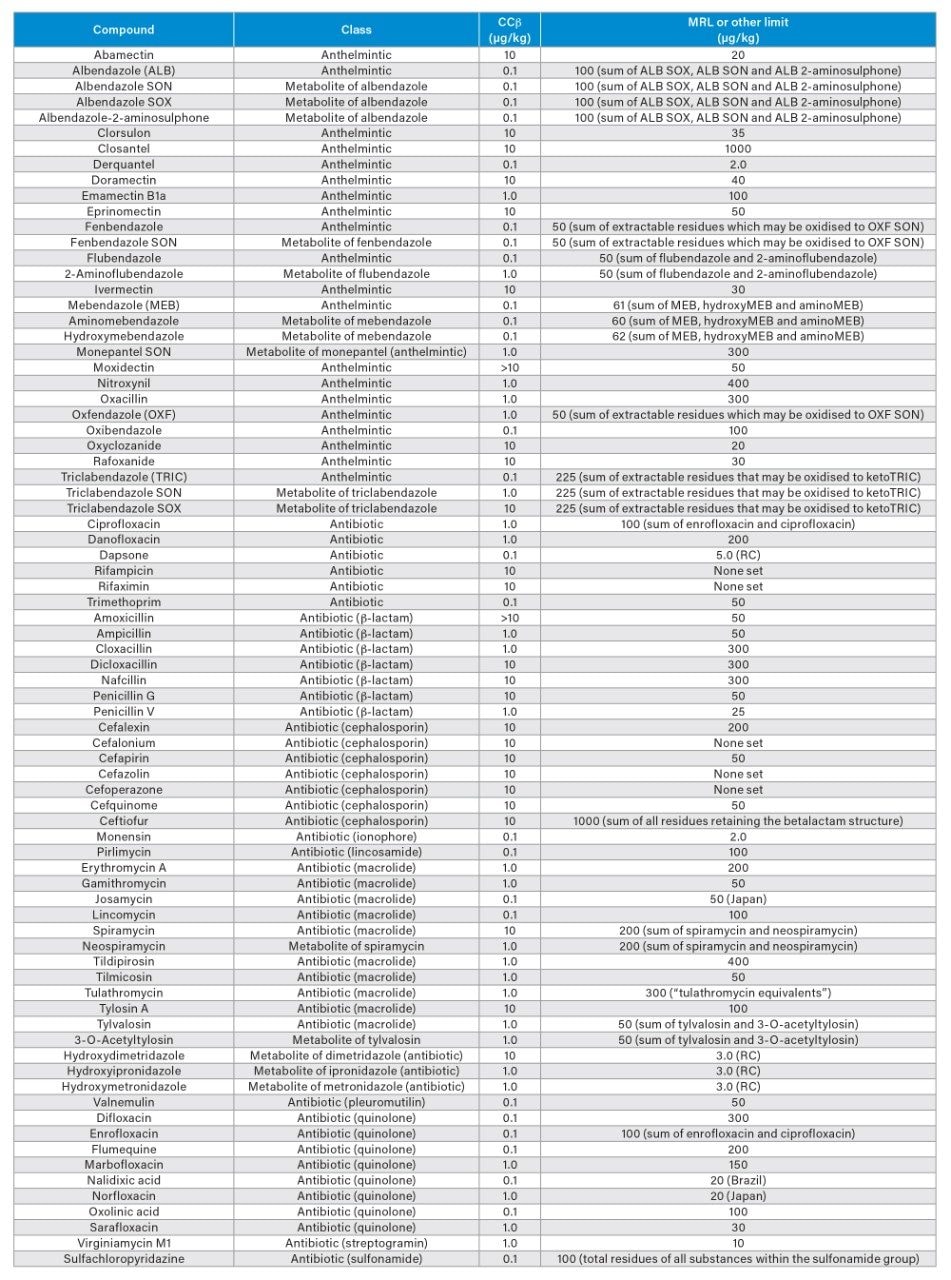
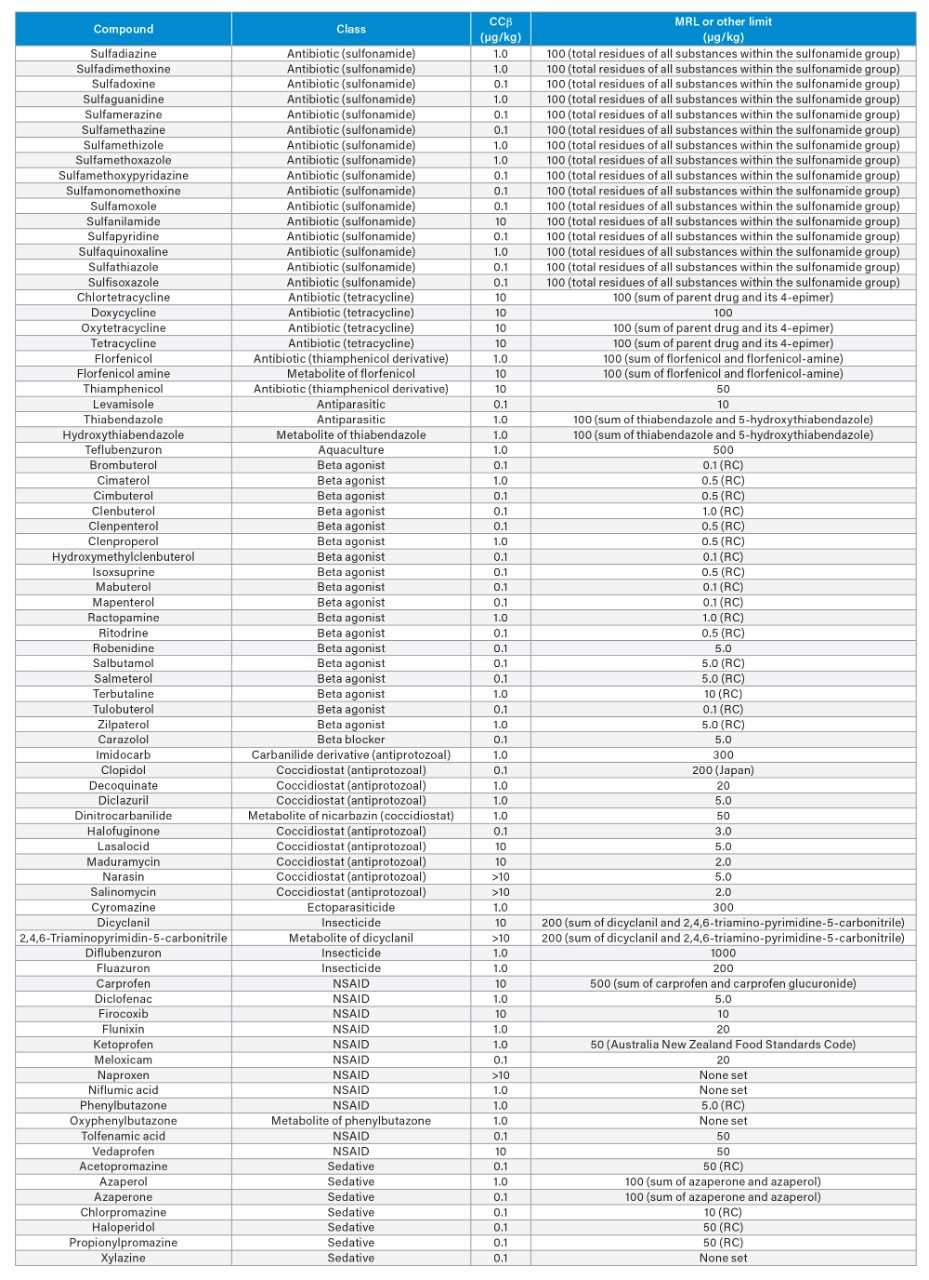
Overall, it was possible to demonstrate validation data in animal muscle tissue for screening for 152 of 158 the analytes tested, with CCβ established at either 0.1 or 1.0 µg/kg for almost 70% of the compounds (Figure 7). The Annex shows a summary of the CCβ values obtained for each analyte along with relevant MRLs. MRLs vary by matrix and species so the lowest available animal tissue MRL was selected for the comparison. For compounds with an established MRL, the CCβ values should be less than or equal to the MRL. In the case of analytes for which no regulatory limit has been established, CCβ must be as low as possible or lower than any MRPL or RC value.
LC-MS/MS has become a powerful technique for screening animal tissues and associated foodstuffs for veterinary drug residues. Here we have presented a multi-residue method that can be used for analysis of animal tissues and related foodstuffs. The method provides rapid extraction and clean-up combined with the sensitivity and selectivity of UPLC and MS/MS. It was successfully validated for screening samples of animal muscle tissue for residues of a wide range of veterinary drug residues at concentrations typically well below MRLs. In most cases, values for CCβ were established at 0.1 or 1.0 µg/kg. As validation criteria have been met the method is considered sensitive, robust, specific, and fit for the purpose of screening for veterinary drug residues.
Scientists must validate the method in their own laboratories and demonstrate that the performance is fit-for-purpose and meets the needs of the relevant analytical control assurance system.
720007116, January 2021