Using the MRM technique a method was developed for the quantification of all four derivatised nitrofuran metabolites in bovine kidney samples. Also compared are the ionisation efficiency of Electrospray and IonSabre APcI along with the sensitivity of the Quattro Ultima Pt and the Quattro micro LC-MS/MS instruments for the determination of the nitrofuran metabolites.
The four drugs furazolidone, furaltadone, nitrofurazone, and nitrofurantoin are veterinary drugs that belong to the family of nitrofuran antibiotics. They have been widely used for the treatment of gastrointestinal infections in cattle, pigs, and poultry. The European Union (EU) banned the use of nitrofuran antibiotics in food-producing animals by listing them in Annex IV of Council Regulation 2377/90.1 No maximum residue limits have been set, so the goal of analytical methods has been to reach the lowest possible limits of detection (LODs). However, the EU has set a Minimum Required Performance Limit (MRPL) of 1 μg/kg that laboratories should at least be able to detect and confirm.
Methods for detecting residues of nitrofuran antibiotics by measuring the parent drugs are inappropriate as they are rapidly metabolised within hours of administration. However, nitrofuran antibiotics form protein-bound metabolites which may persist for weeks and even months. It is possible to release these residues from proteins under moderately acidic conditions.2 The structures of the nitrofuran antibiotics and their free metabolites are shown in Figure 1.
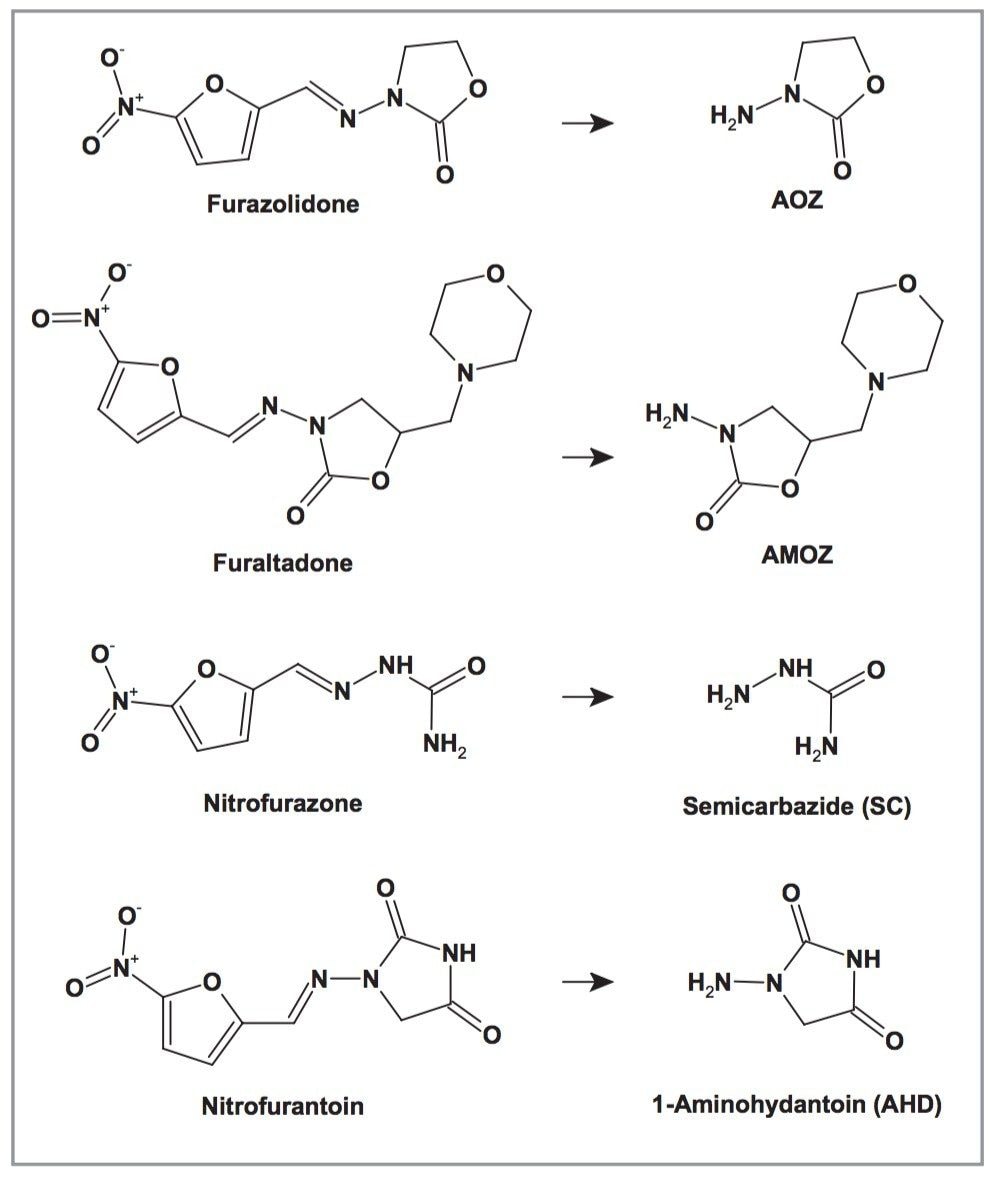
All of the nitrofuran metabolites have molecular masses between 75 and 201. Due to more abundant background noise in this mass range, a low ionisation efficiency of the analytes and their non-specific fragmentation behaviour (predominantly loss of ammonia, water or carbon dioxide), the MS sensitivity is relatively poor. Consequently, derivatisation of the free amino group of the target analytes with 2-nitrobenzaldehyde is normally carried out to achieve compounds with more favourable properties.
Due to the low limits of detection required by the MRPL, a significant concentration factor is used in the extraction method. This increases the potential for matrix interference during the determinative step. Triple quadrupole mass spectrometry in the Multiple Reaction Monitoring (MRM) mode provides the analytical selectivity required for achieving low analyte detection levels in complex sample matrices and was employed in accordance with European Union guidelines.3
Using the MRM technique a method was developed for the quantification of all four derivatised nitrofuran metabolites in bovine kidney samples. Also compared are the ionisation efficiency of Electrospray and IonSabre APcI along with the sensitivity of the Quattro Ultima Pt and the Quattro micro LC-MS/MS instruments for the determination of the nitrofuran metabolites.

The bovine kidney extracts were supplied by CVUA Münster, Germany.
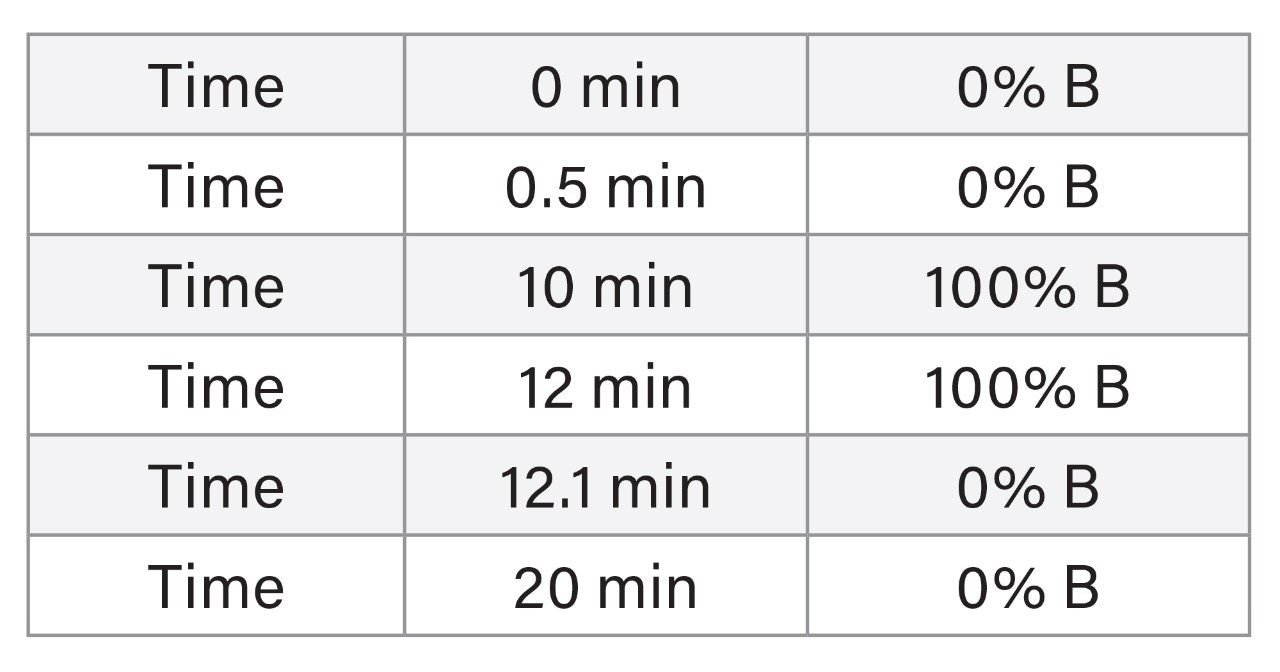
|
LC system: |
Alliance 2795 HPLC System |
|
Mobile phase A: |
Methanol/Water (1:4, v/v) + 5 mM CH3CO2NH4 |
|
Mobile phase B: |
Methanol/Water (9:1, v/v) + 5 mM CH3CO2NH4 |
|
Column: |
XTerra, 2.1 x 100 mm, 5 μm at 30 °C |
|
Flow rate: |
0.2 mL/min |
|
Injection volume: |
20 μL |
Waters Micromass Quattro Ultima Pt
Electrospray and IonSabre APcI modes with positive polarity.
|
Capillary voltage: |
1 kV |
|
RF lens 1: |
55 V |
|
Aperture: |
0 V |
|
RF lens 2: |
0 V |
|
Source temp.: |
120 °C |
|
Desolvation temp.: |
450 °C |
|
Cone gas flow: |
100 L/hr |
|
Desolvation gas flow: |
700 L/hr |
|
Multiplier: |
650 V |
|
Corona current voltage: |
25 μA |
|
RF lens 1: |
50 V |
|
Aperture: |
0 V |
|
RF lens 2: |
0 V |
|
Source temp.: |
120 °C |
|
IonSabre APcI probe temp.: |
500 °C |
|
Cone gas flow: |
100 L/hr |
|
Cone gas flow: |
700 L/hr |
|
Multiplier: |
650 V |
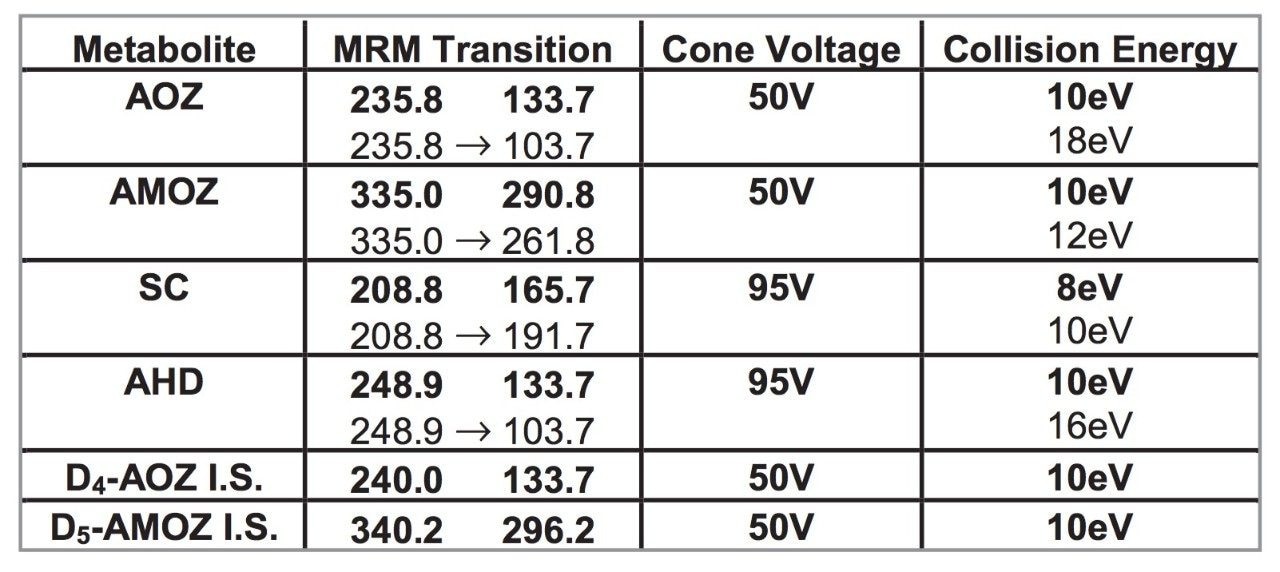
The MRM transitions along with the cone voltages and collision energies are listed in Table 1. For each derivatised nitrofuran metabolite, two MRM transitions were chosen, one as the quantification transition (bold type) and one as the confirmation transition (regular type) in accordance with European guidelines.3 These transitions optimised with argon as the collision gas at a pressure of 4.5e-3 mBar. D4-AOZ and D5-AMOZ were used as internal standards, providing information on the amount of suppression in the kidney extracts. A dwell time of 0.1 s was used for each transition, which equates to approximately 14 points per chromatographic peak.
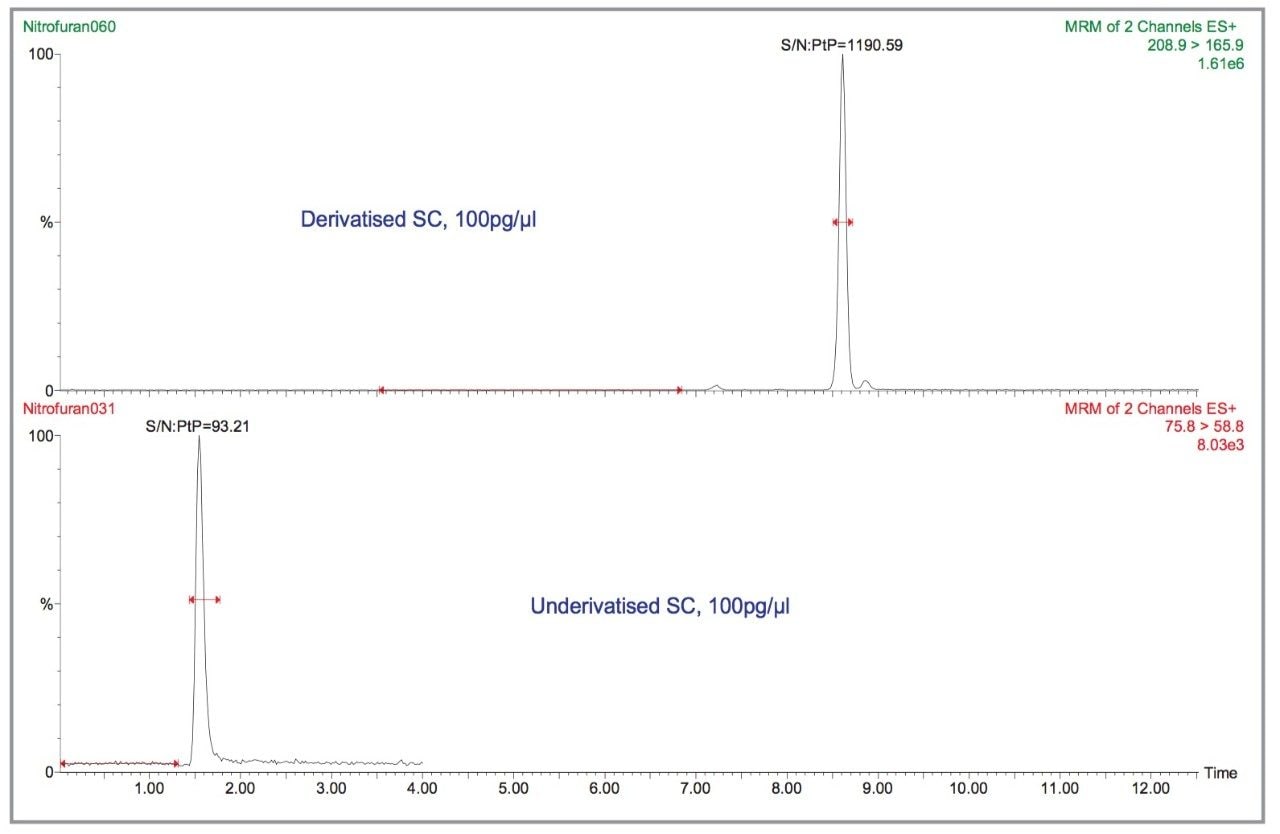
The derivatisation of the nitrofuran metabolites involves the reaction between 2-nitrobenzaldehyde and the free amino group of the protein-bound metabolite under acidic conditions. Extraction is generally performed by acid hydrolysis, which takes 16 hours so derivatisation does not increase this time or add any complexity. Derivatisation also aids extraction as once the metabolite is derivatised it can no longer bind to the protein. The difference in sensitivity between derivatised and underivatised SC is illustrated in Figure 2. The other metabolites do not give the factor of ten difference shown by SC; but in general, the derivatised metabolite gives more sensitivity than the underivatised one. From this point on, only the derivatised metabolites will be considered.
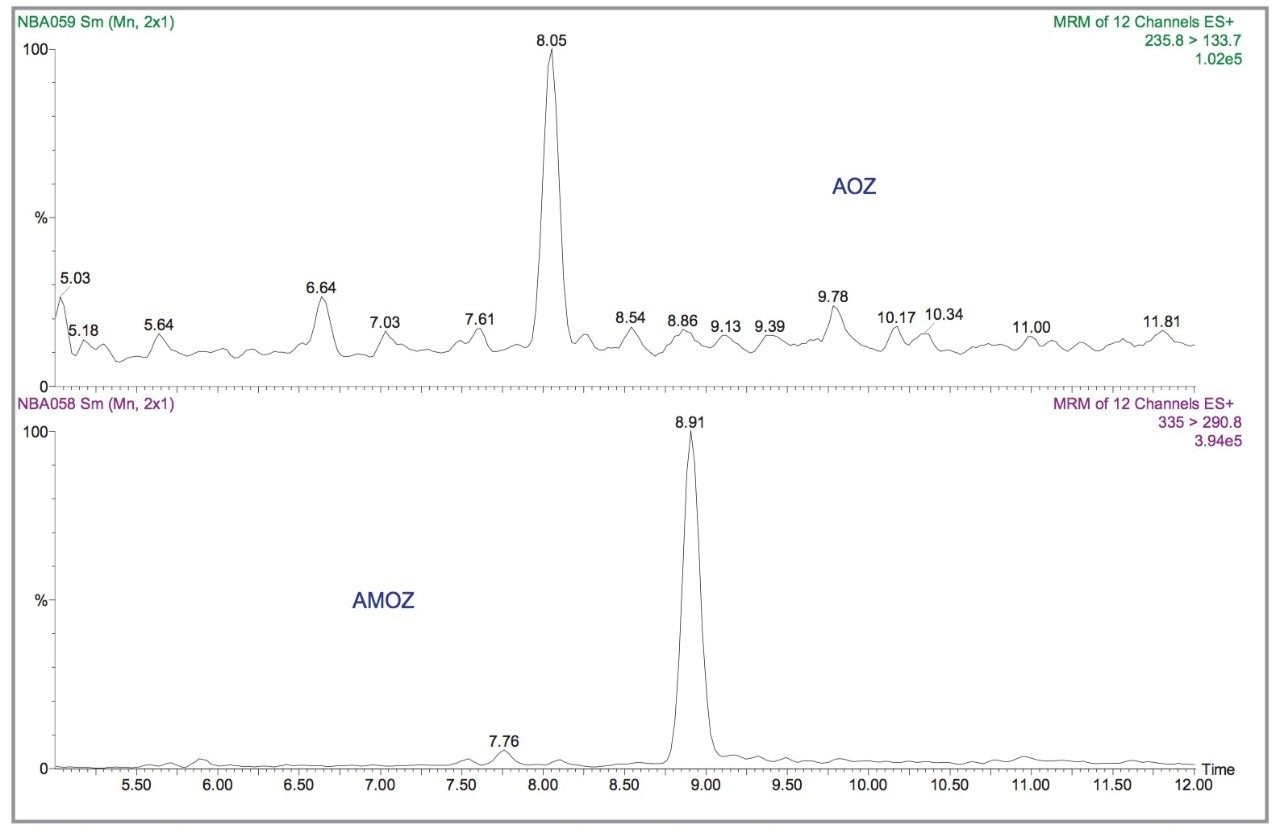
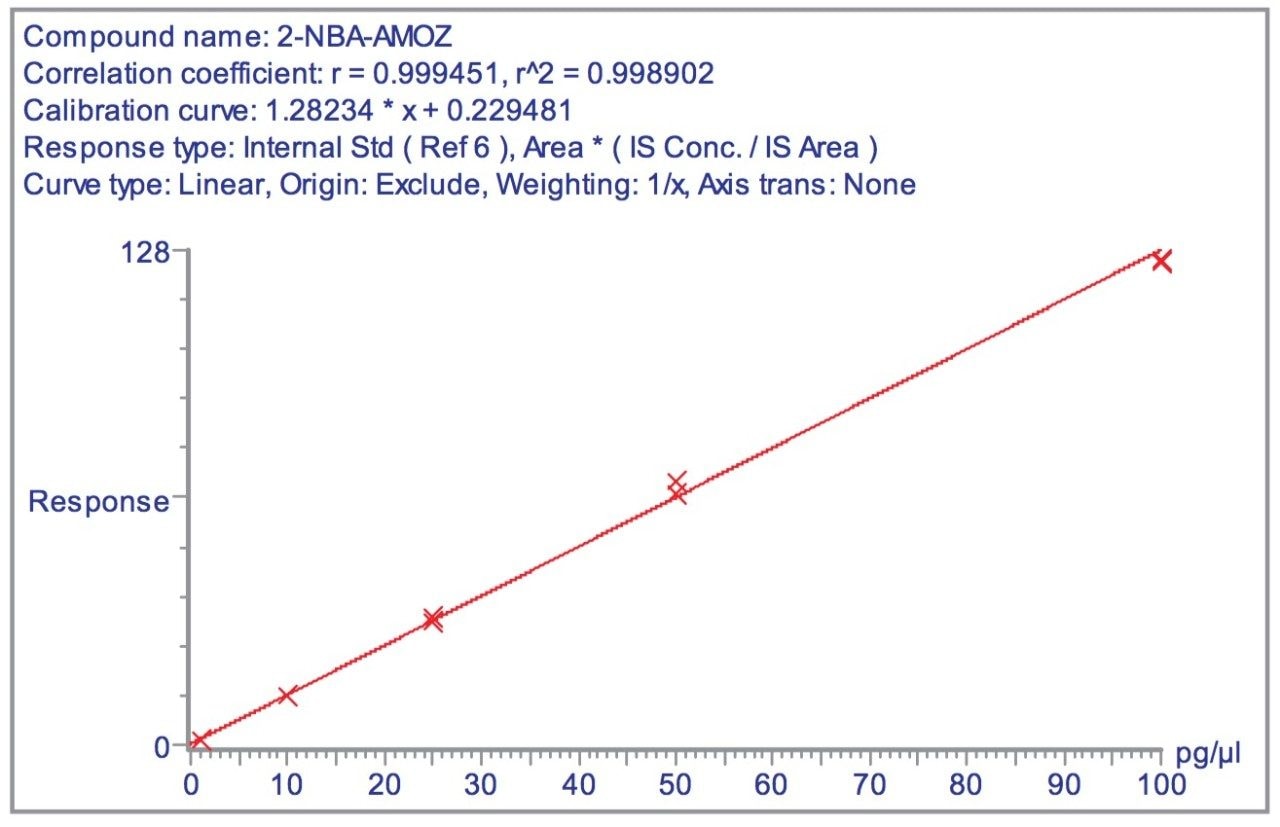
In Electrospray mode, the MRM transitions of the 1 pg/μL standard for AOZ and AMOZ are illustrated in Figure 3. From the figure, the LODs in solvent standards (S/N ratio= 3:1) for AOZ and AMOZ are estimated to be 0.25 and 0.05 pg/μL, respectively. Figure 4 shows a representative calibration curve for AMOZ generated in the concentration range 1–100 pg/μL. Calibration was performed using the D5 analogue of AMOZ as the internal standard.
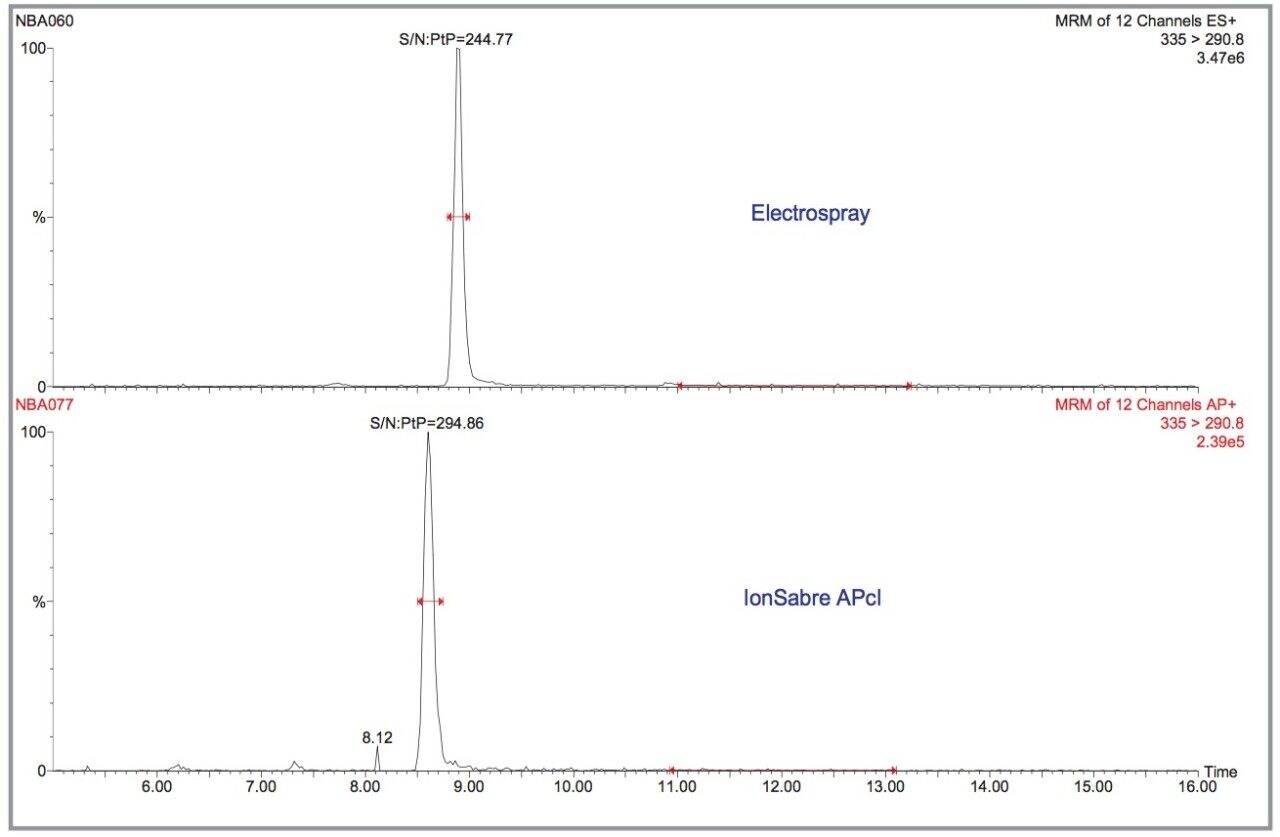
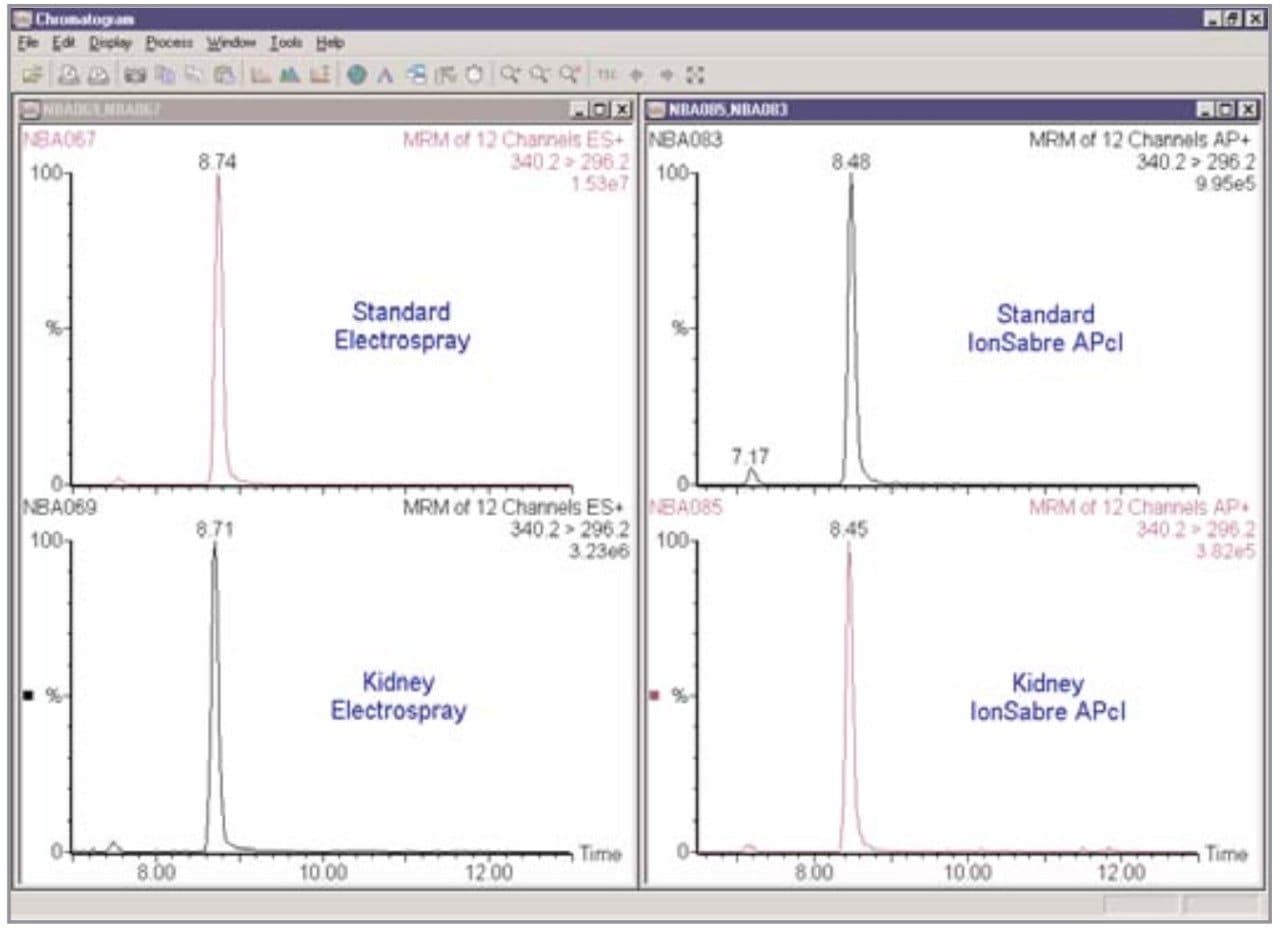
The sensitivity of Electrospray and IonSabre APcI for the nitrofuran metabolites was compared. IonSabre APcI should be particularly useful for the extracted residues, where the amount of coextractive interference from kidney, may affect the Electrospray response more than APcI. The difference between Electrospray and IonSabre APcI can be observed in Figure 5 for 10 pg/μL AMOZ. In terms of the signal produced, Electrospray is an order of magnitude more sensitive than APcI, but comparing the S/N ratios shows that the two techniques are comparable.
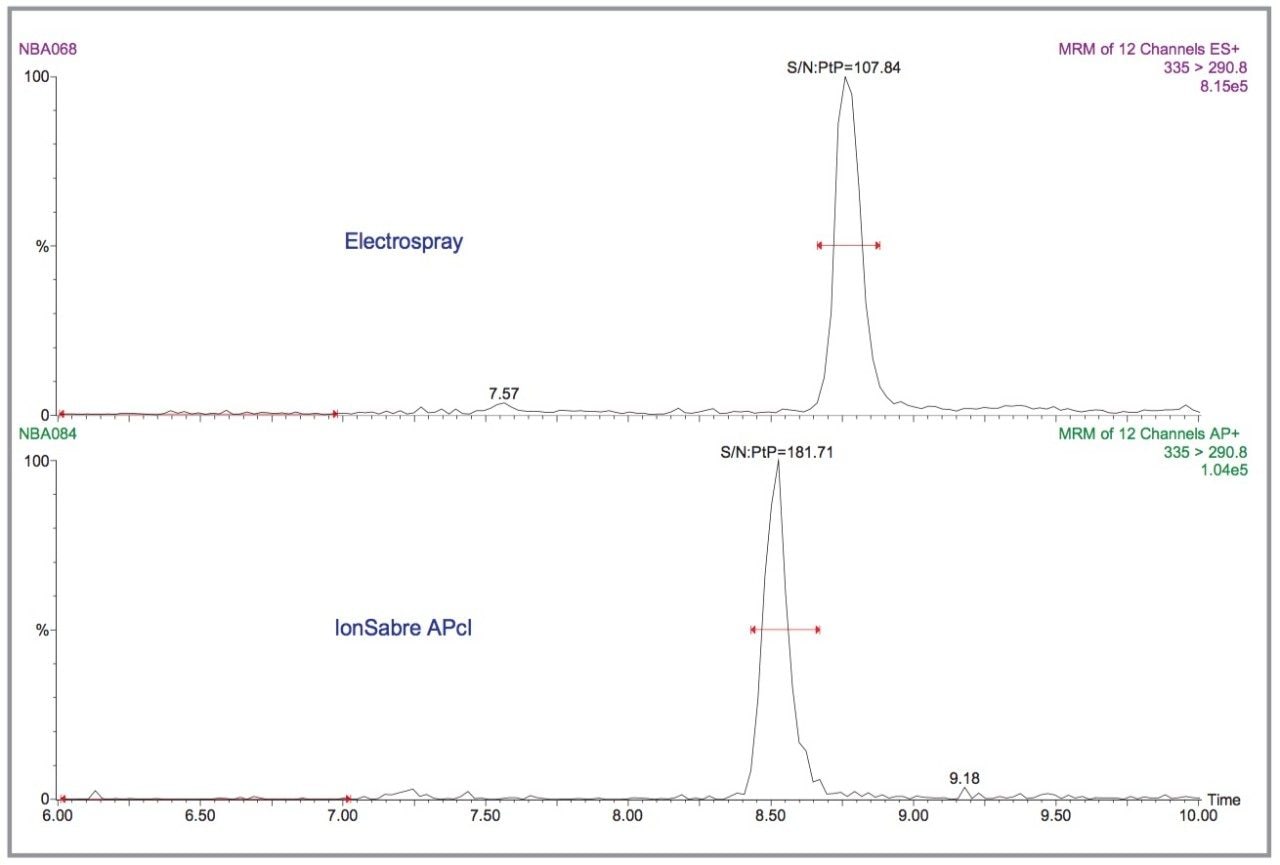
The same can be said for kidney samples spiked at the 0.9 mg/kg concentration level (Figure 6).
In IonSabre APcI mode, the MRM transitions of the 1 pg/μL standard for AOZ and AMOZ are illustrated in Figure 7. From the figure, the LODs in solvent standards (S/N ratio= 3:1) for AOZ and AMOZ are estimated to be 0.1 and 0.04 pg/μL, respectively.
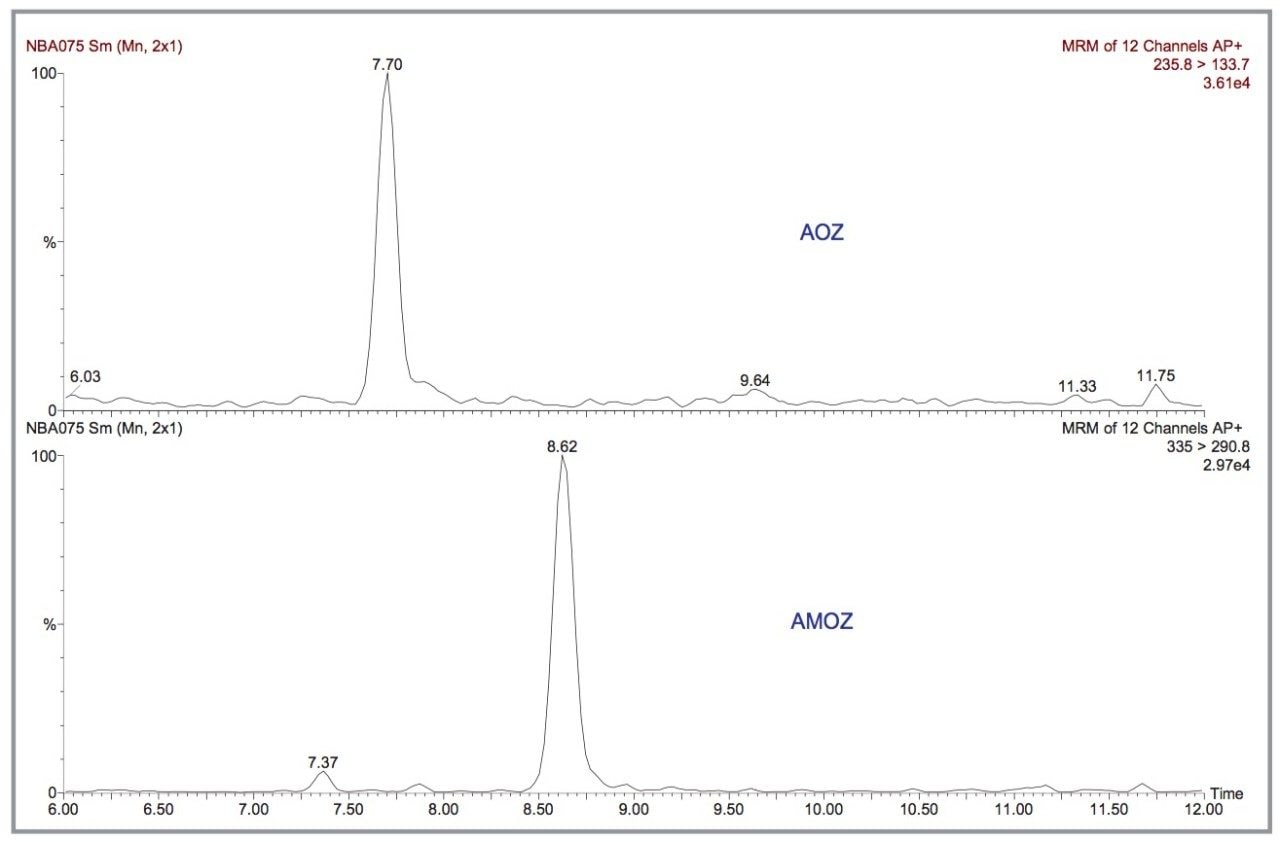
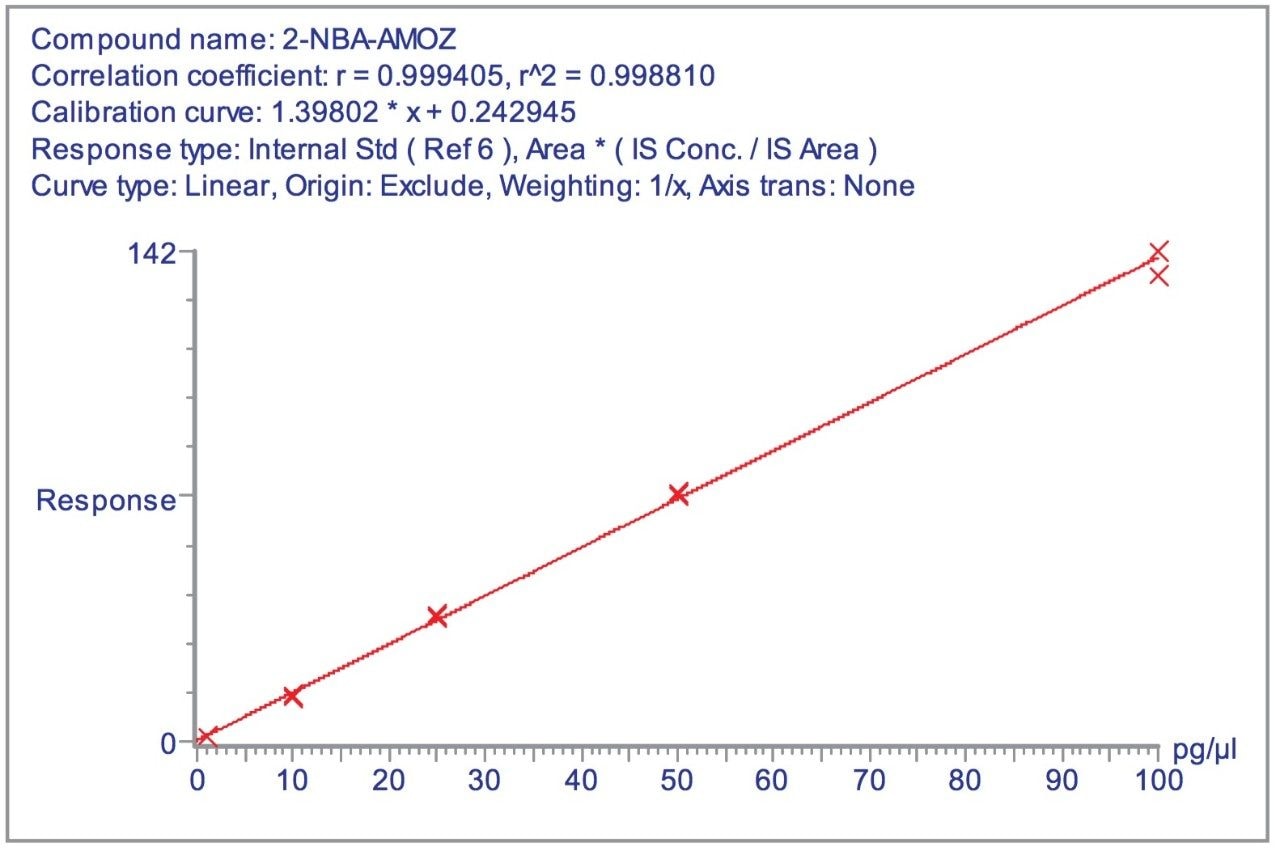
Figure 8 shows a representative calibration curve for AMOZ generated in the concentration range 1–100 pg/μL. Calibration was performed using the D5 analogue of AMOZ as the internal standard.
The matrix interference that leads to suppression is illustrated in Figure 9 using the D5 analogue of AMOZ. Here, the amount of signal from a solvent standard is compared to that from a spiked kidney sample. The signal is reduced by approximately a factor of five between the standard and the sample due to matrix interference for Electrospray mode; for IonSabre APcI this reduction is only a factor of three. This confirms the assumption that APcI mode is less affected by coextractive interference than Electrospray for this particular analysis.
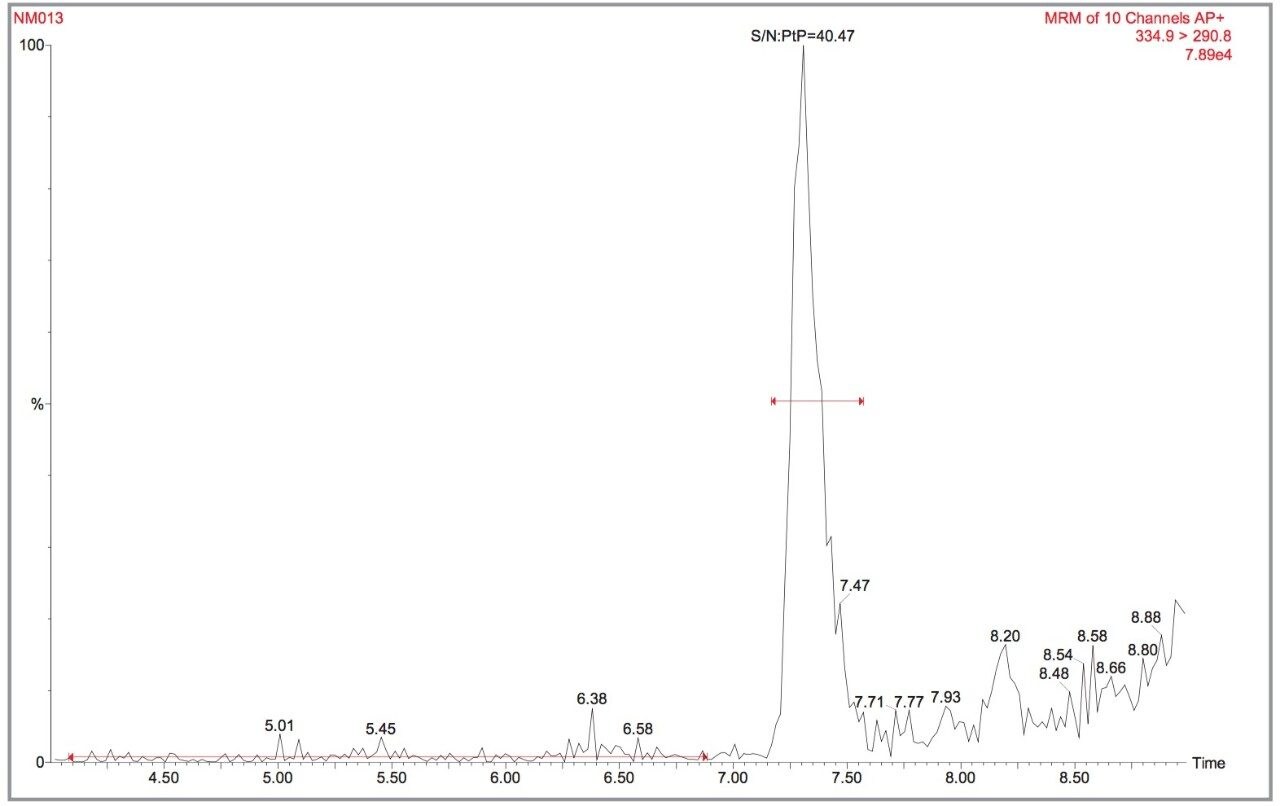
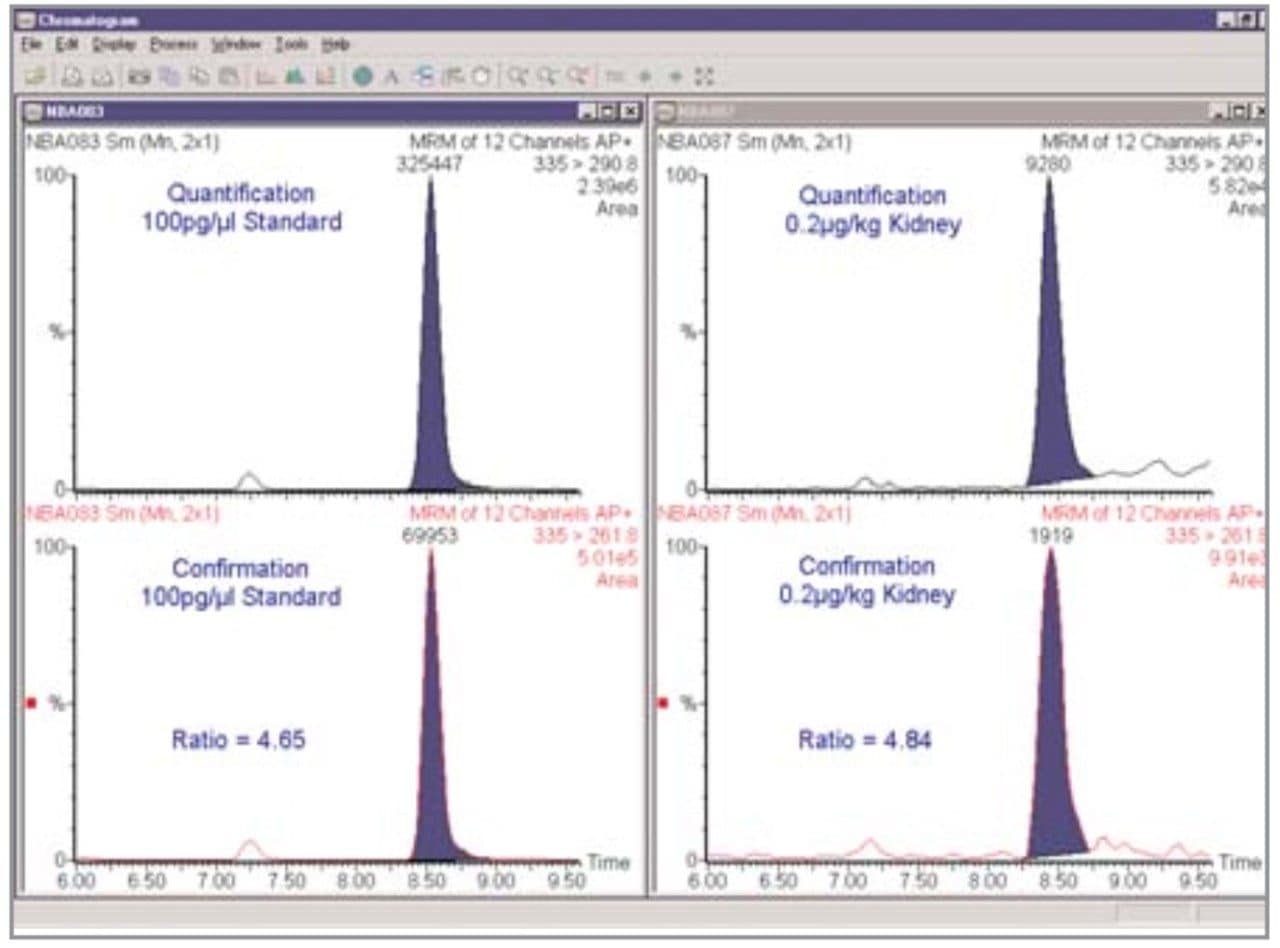
The lowest spiked concentration kidney sample supplied was 0.2 μg/kg AMOZ, as illustrated in Figure 10. A S/N ratio of 40:1 in IonSabre APcI mode for 20 μL injected was obtained. By extrapolation this would lead to an LOD (S/N ratio = 4:1) of 0.02 μg/kg AMOZ. These results indicate LODs much lower than the MRPL of 1.0 μg/kg are achievable with the Quattro Ultima Pt.
A second confirmatory MRM transition for all the nitrofuran metabolites was also monitored in accordance with EU guidelines.3 A peak area ratio of 4.65 between the quantification and confirmatory transitions for AMOZ is illustrated in Figure 11 for a 100 pg/μL standard. A peak area ratio of 4.84 was obtained for the 0.2 μg/kg spiked kidney sample. The measured 4% difference is well inside the ±25% that the peak area ratio should be for a confirmatory transition that is between 20 and 50% intensity of the quantification transition.
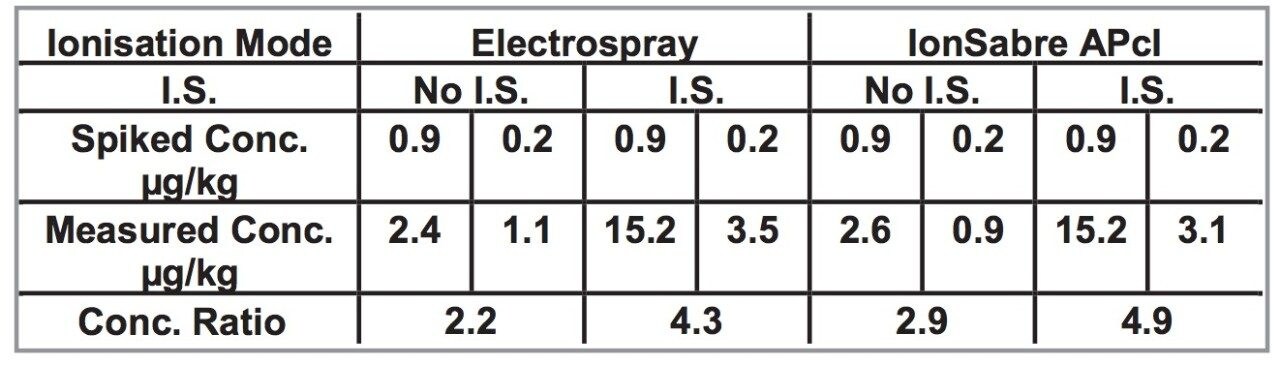
It has already been hinted that there are significant matrix effects from coextractive interference but are internal standards really needed? For the spiked kidney samples, two concentrations of AMOZ were supplied in the ratio 9:2. The results listed in Table 2 show that the measured concentration ratio using internal standards for both Electrospray (4.3) and IonSabre APcI (4.9) are similar to that expected (4.5) while the measured concentration ratio without internal standards does not compare.
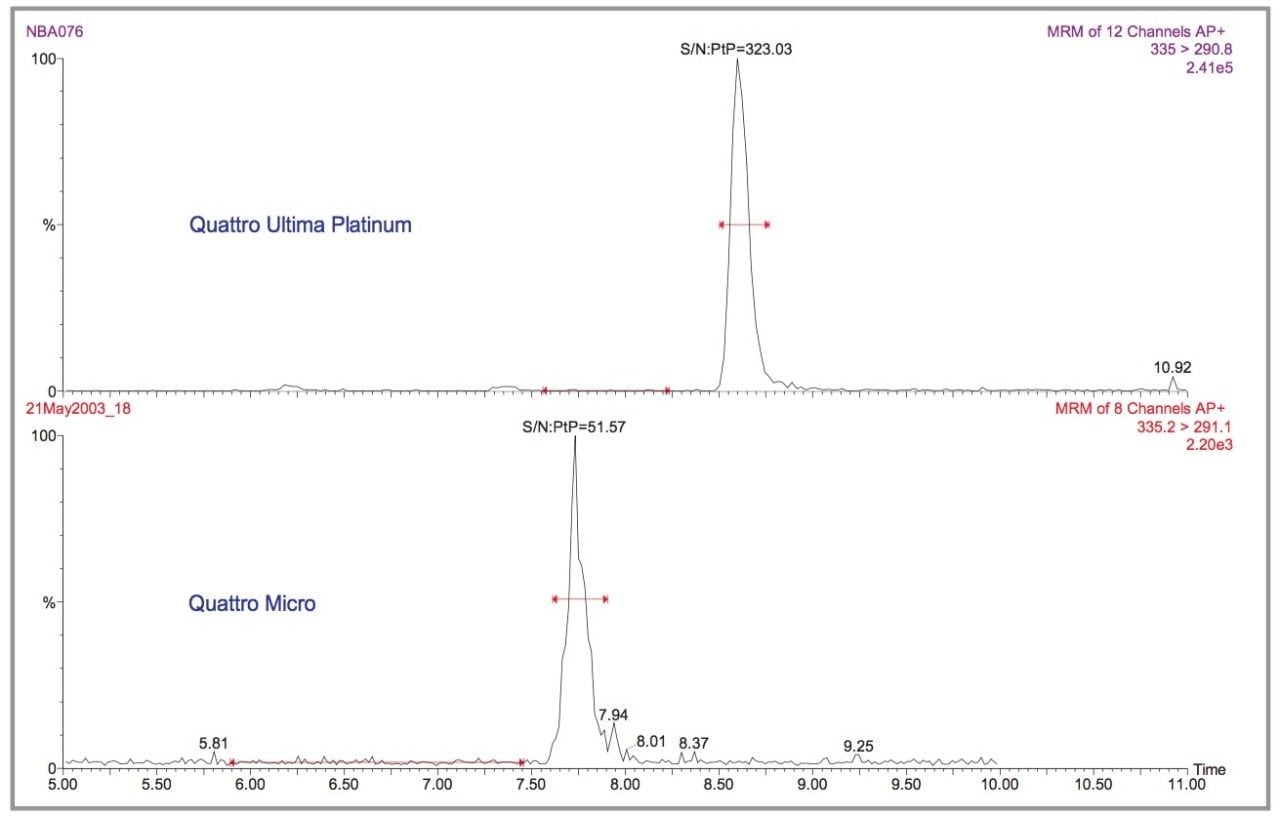
Finally, the difference in sensitivity between the Quattro Ultima Pt and the Quattro micro was investigated and is illustrated in Figure 12. For AMOZ the difference was approximately a factor of 6. From the extrapolated LOD of 0.02 μg/kg for the Quattro Ultima Pt, this leads to a LOD of 0.1–0.2 μg/kg for the Quattro micro. This is still below the MRPL of 1.0 μg/kg but nitrofurans are banned substances so should be completely absent from food products. Therefore, the analytical goal is still to reach the lowest possible detection limits.
A sensitive and selective MRM method has been introduced for the analysis of derivatised nitrofuran metabolites in bovine kidney using the Quattro Ultima Pt. The method allows LODs of 0.02 μg/kg AMOZ to be obtained, fifty times lower than the Minimum Required Performance Limit (MRPL). IonSabre APcI has been shown to be as sensitive as Electrospray and this technique may offer advantages in terms of less coextractive interference affecting results. The method can also be transferred to the Quattro micro allowing LODs of 0.1–0.2 μg/kg AMOZ.
720000705, October 2003