
Rapid, reliable, and cost-effective methods are required by food manufacturers and ingredient suppliers in order to verify product consistency and ensure that label claims are met. This can be a challenging task with the combination of complex matrices and low fortification levels of some vitamins. In addition, many of the methods currently employed stipulate that the vitamins are either analyzed separately, or in small groups. Established techniques include microbiological assays, colorimetric and fluorimetric analysis, titrimetric procedures and HPLC methodologies. LC-MS offers the opportunity to consolidate methods along with the ability to improve detector selectivity and reduce limits of quantification. In order to offer laboratories the opportunity to capture the benefits of mass detection without the challenges associated with the adoption of mass spectrometers, recent advances in technology have focused on improving instrument usability and robustness. These motivations have resulted in the introduction of the ACQUITY QDa Detector. In this application note, 12 water soluble vitamins (WSVs) were analyzed in dietary supplements and beverage samples using the ACQUITY UPLC H-Class System with t he ACQUITY QDa Detector.
Many food and beverage products are routinely fortified with vitamins to enhance their nutritional value and to help address any deficiencies in dietary requirements. In order to meet legal requirements, manufacturers must label their products according to the regulations of the country in which the product is consumed. Examples of these regulations include European Commission (EC) 1925/2006 on the addition of vitamins and minerals, and Title 21 Code of Federal Regulations (C.F.R.), Part 101 on food labeling in the United States.
Rapid, reliable, and cost-effective methods are required by food manufacturers and ingredient suppliers in order to verify product consistency and ensure that label claims are met. This can be a challenging task with the combination of complex matrices and low fortification levels of some vitamins. In addition, many of the methods currently employed stipulate that the vitamins are either analyzed separately, or in small groups. Established techniques include microbiological assays, colorimetric and fluorimetric analysis, titrimetric procedures and HPLC methodologies.1 LC-MS offers the opportunity to consolidate methods along with the ability to improve detector selectivity and reduce limits of quantification. Waters ACQUITY QDa Mass Detector offers laboratories the opportunity to capture the benefits of mass detection without the challenges associated with the adoption of mass spectrometers.
In this application note, 12 water soluble vitamins (WSVs) were analyzed in dietary supplements and beverage samples using the ACQUITY UPLC H-Class System with the ACQUITY QDa Mass Detector.
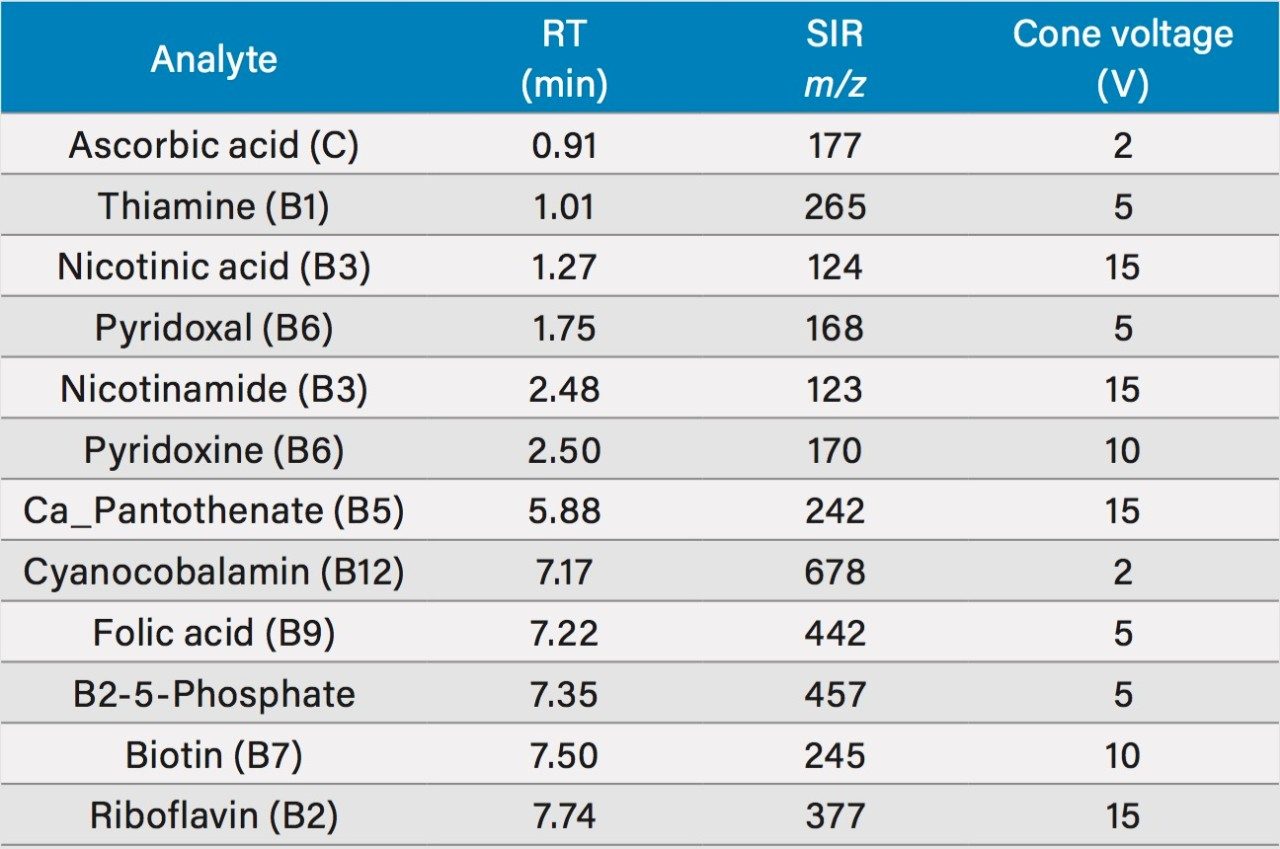
Table 1 lists the water soluble vitamins included in this study along with the observed retention times, single ion recording (SIR) m/z, and cone voltage.
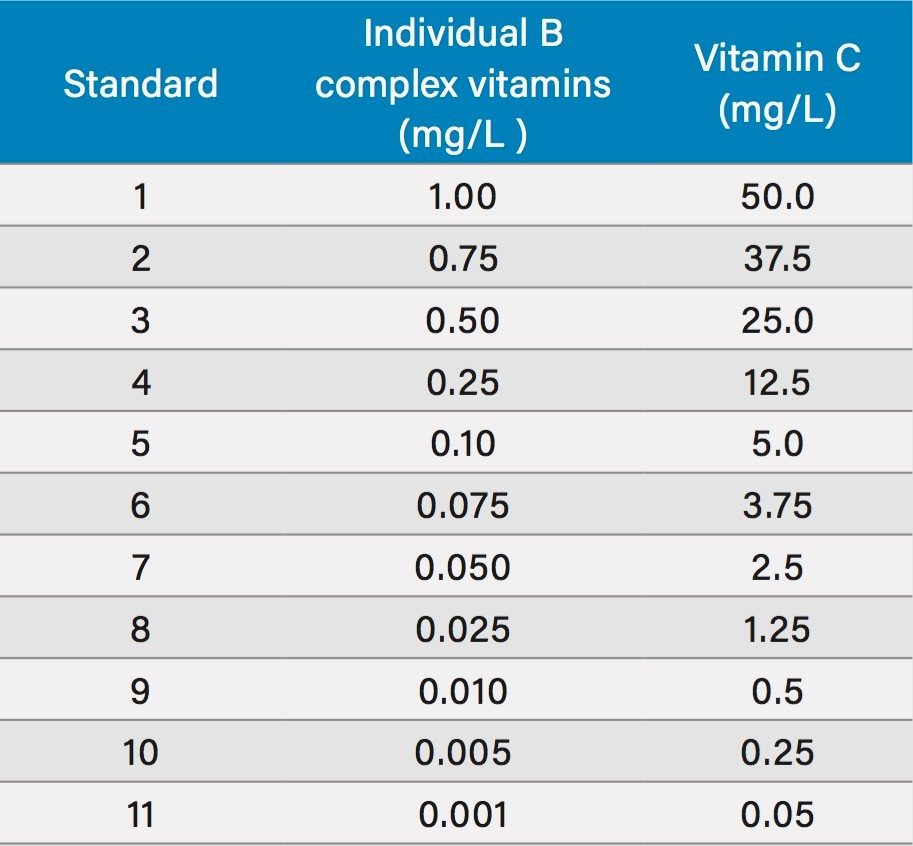
Individual 1 mg/mL WSV stocks were prepared in water. In the case of vitamins B2, B7, and B9, 200 μL of 1 N NaOH were added to affect dissolution. Vitamin C was dissolved in a low pH acetate buffer to enhance stability. From these individual stocks, a mixed stock was prepared by adding 1.25 mL of the vitamin C stock and 0.025 mL of the other stocks and diluting to 25 mL with water. This mixed stock (50 ppm vitamin C, 1 ppm of the other analytes) was further diluted to provide 11 individual calibration standards, listed in Table 2.
A packet (8.50 g) of a powdered vitamin beverage was dissolved in 100 mL water and filtered through a 0.2-μm PVDF filter. This sample was then prepared at two additional dilution levels: 1:250 and 1:10. These three dilution levels were injected to cover the different concentrations of vitamins in this sample.
A multi-vitamin supplement tablet was crushed using a mortar and pestle. The powder (1.34 g) was quantitatively transferred to a beaker to which 100 mL water was added. This mixture was sonicated for 15 minutes then stirred and filtered through a 0.2-μm PVDF filter. Three additional dilutions of this sample were prepared in water: 1:1000, 1:100, and 1:20. These dilutions and the initial dissolved tablet solution (undiluted) were analyzed in order to cover the different concentrations of vitamins in this sample.
Two different vitamin water samples were prepared by diluting 1:20 with water and filtering through a 0.2-μm PVDF filter.
|
UPLC system: |
ACQUITY UPLC H-Class |
|
Run time: |
17.5 min |
|
Column: |
ACQUITY UPLC HSS T3, 1.8 μm, 2.1 x 100 mm |
|
Column temp.: |
30 °C |
|
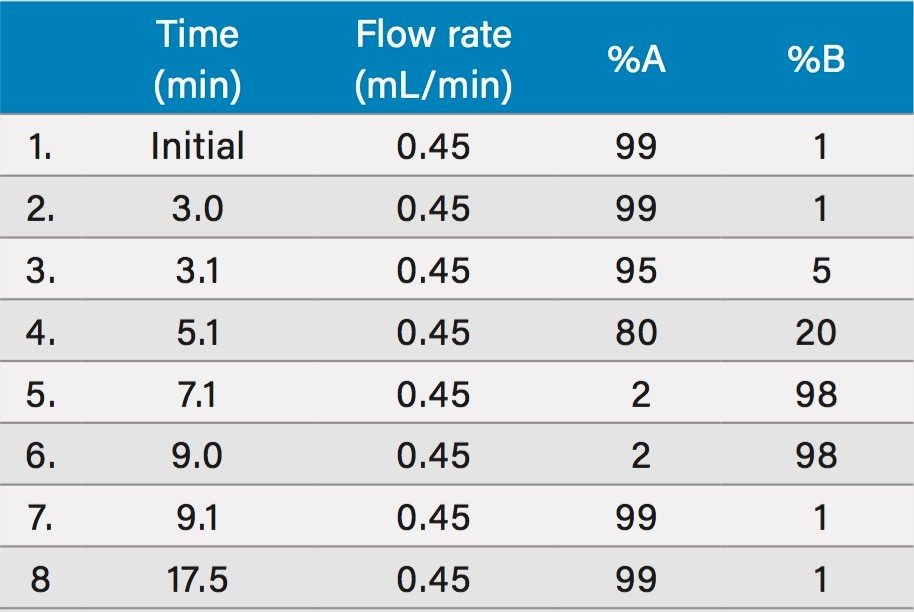
Mobile phase A: |
10 mM ammonium formate, 0.1% formic acid in water |
|
Mobile phase B: |
10 mM ammonium formate, 0.1% formic acid in methanol |
|
Injection volume: |
5 μL |
|
Detector 1: |
ACQUITY UPLC PDA |
|
Wavelength: |
Scanning 210 to 400 nm; Analog channel at 270 nm |
|
Scan rate: |
10 pts/sec |
|
Detector 2: |
ACQUITY Qda |
|
Ionization mode: |
ESI+ |
|
Run time: |
8.0 min |
|
Probe temp.: |
600 °C |
|
Capillary voltage: |
0.8 kV |
|
Mass range: |
m/z 50 to 800 (centroid) and select SIRs* |
|
Sampling freq.: |
5 Hz |
|
Cone voltage: |
Full scan data: 15 V *See Table 1 for cone voltage of individual SIR channels. SIR m/z were assigned based on previous work.2 |
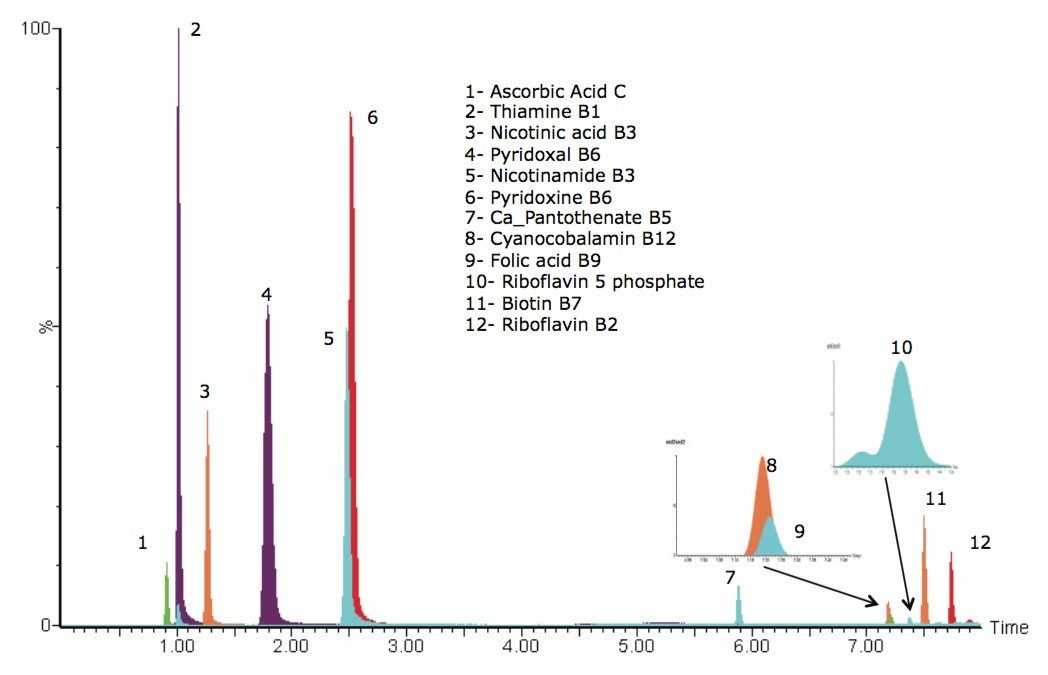
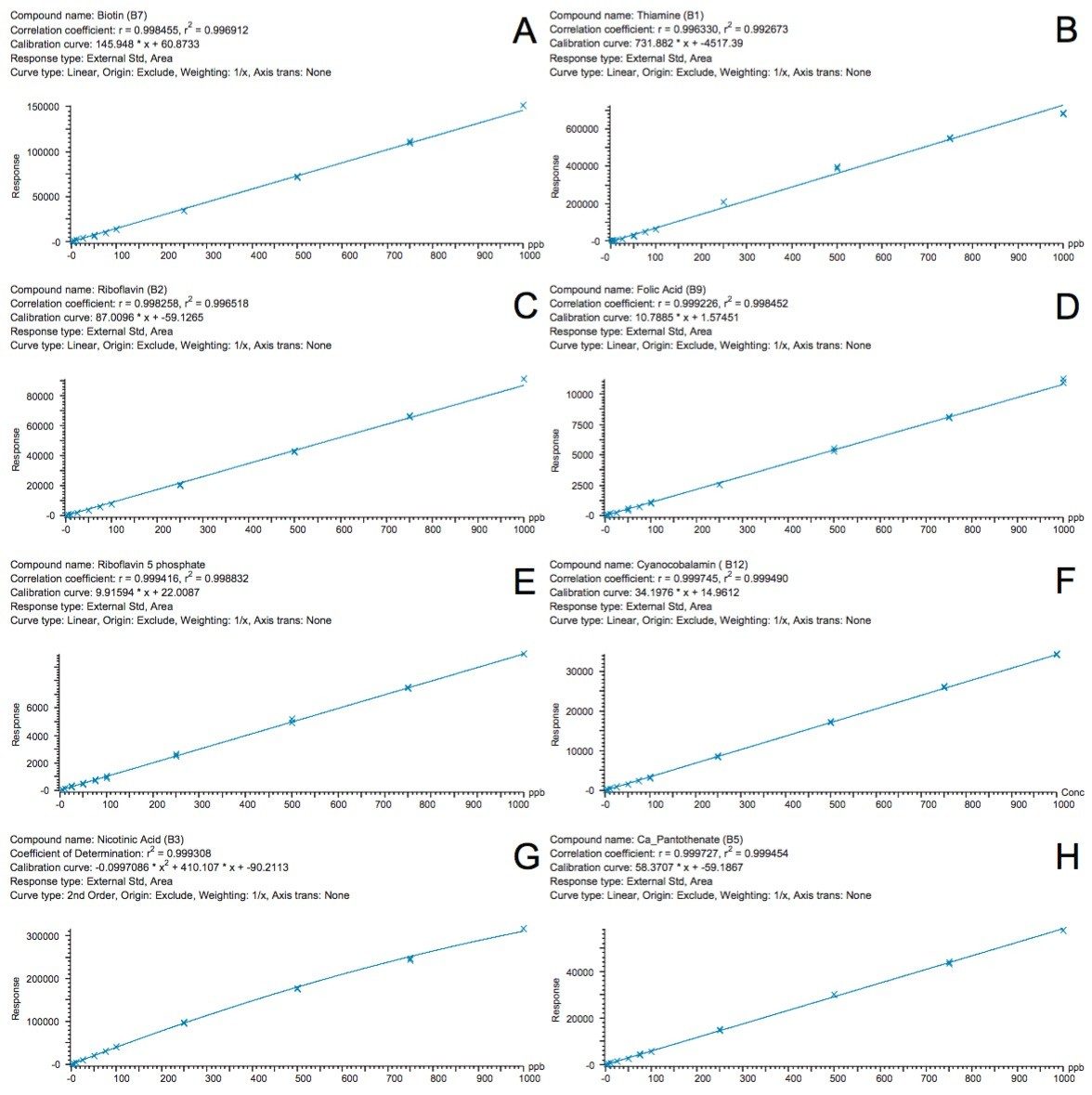
A chromatogram showing an overlay of all 12 water soluble vitamins used in this study is shown in Figure 1, where all compounds eluted within eight minutes. Using this method, there were two co-eluting pairs (nicotinamide and pyridoxine at ~2.5 minutes and cyanocobalamin and folic acid at ~7.25 minutes). The use of mass detection means that it is no longer necessary to ensure baseline separation of all the analytes. The discrimination offered with mass detection means that these compounds can be accurately measured using their mass-to-charge ratio (m/z). This is demonstrated in Figure 2 where the linearity of selected vitamins are shown, including vitamins that co-eluted. Figure 2D and 2F show the calibration curves of folic acid (m/z 442) and cyanocobalamin (m/z 678), respectively. The selectivity offered with mass detection means that these compounds can be determined quantitatively, even though they co-elute. Figure 2 also shows example calibration curves of vitamins that can be challenging to analyze by UV. For example, biotin (Figure 2A) and calcium pantothenate (Figure 2H) are vitamins that show low responses using UV detection. Those compounds are often analyzed at low wavelengths to obtain a sufficiently sensitive response.3 At such low wavelengths, the specificity of the analysis may be compromised. Mass detection ensures that the analysis is both specific and sensitive.
Mass detection offers the opportunity to detect vitamins at lower levels than can be achieved with UV detection. In Figure 3, the SIR chromatograms of vitamins pyridoxine, pyridoxal, nicotinic acid, and nicotinamide at 5 ppb (5 μg/L) are shown, along with the UV chromatogram (Figure 3A, 270 nm). As shown in Figure 3A, the vitamins could not be detected by UV at this level. The lower limits of quantification that can be achieved with mass detection is important for the quantification of vitamins at low levels. Improved sensitivity also helps to deal with the wide variety of matrices that are encountered by allowing sample extracts to be diluted. In this work, vitamin supplements and drinks were analyzed simply by diluting the sample (in the case of a tablet, an initial step to crush the tablet was required).
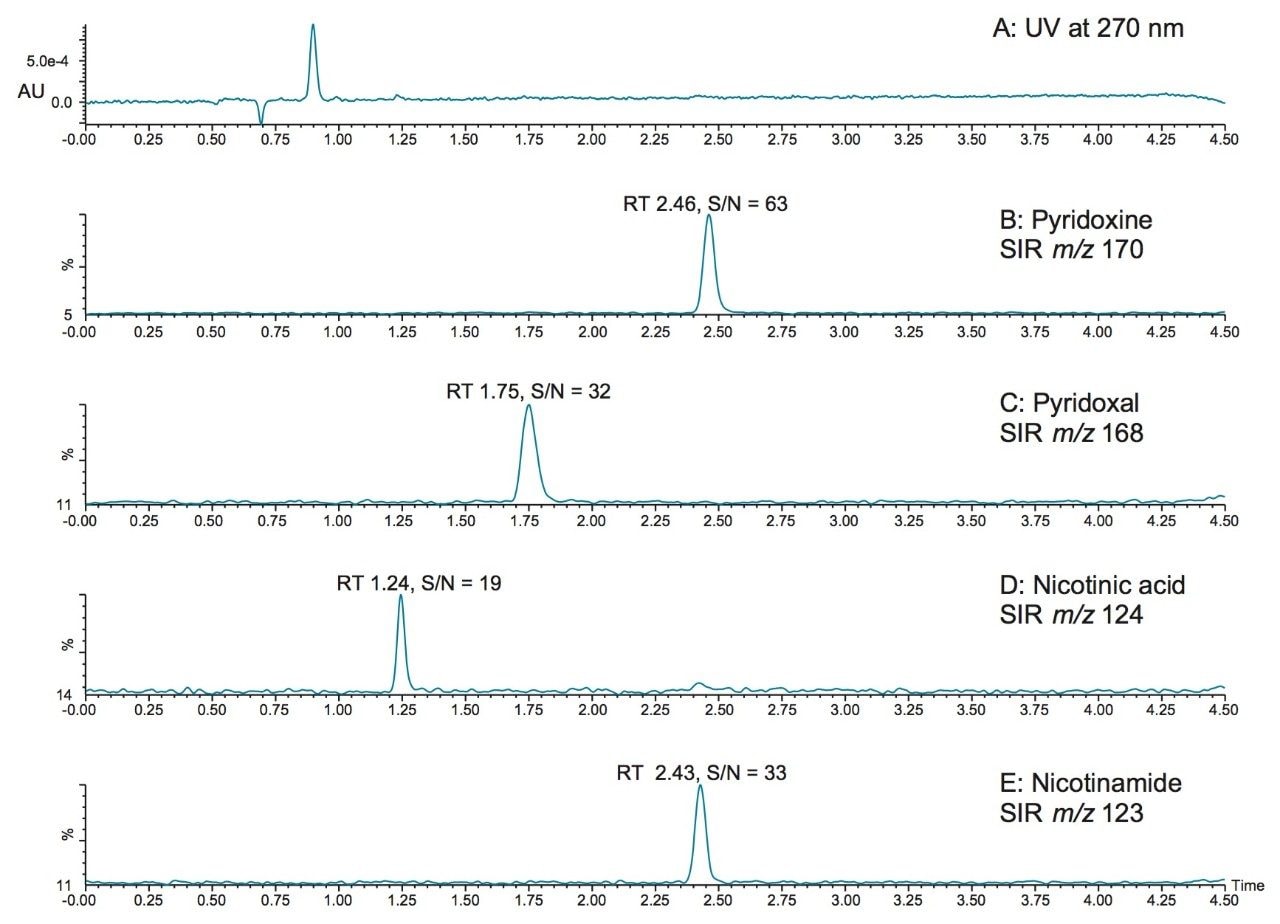
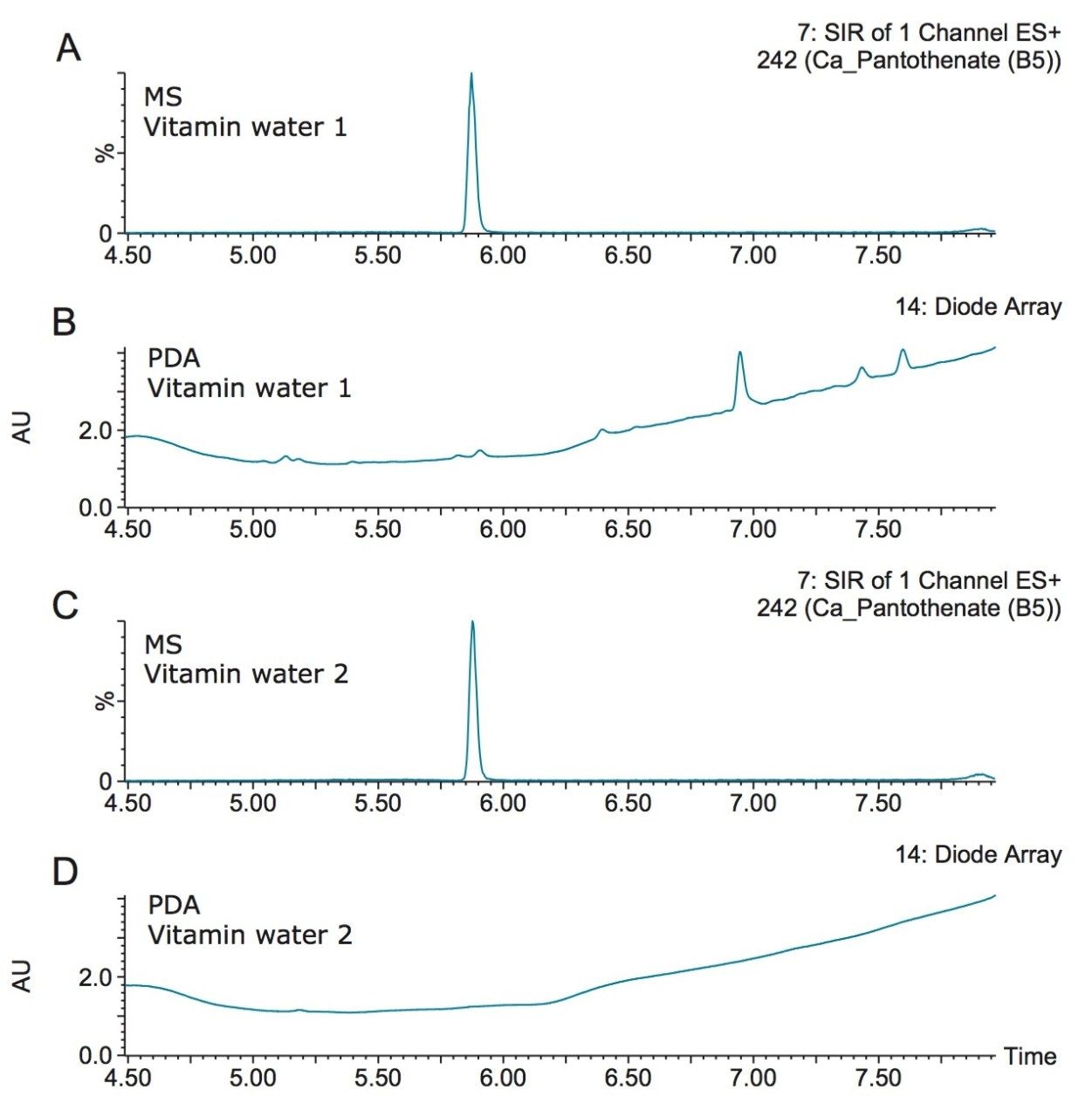
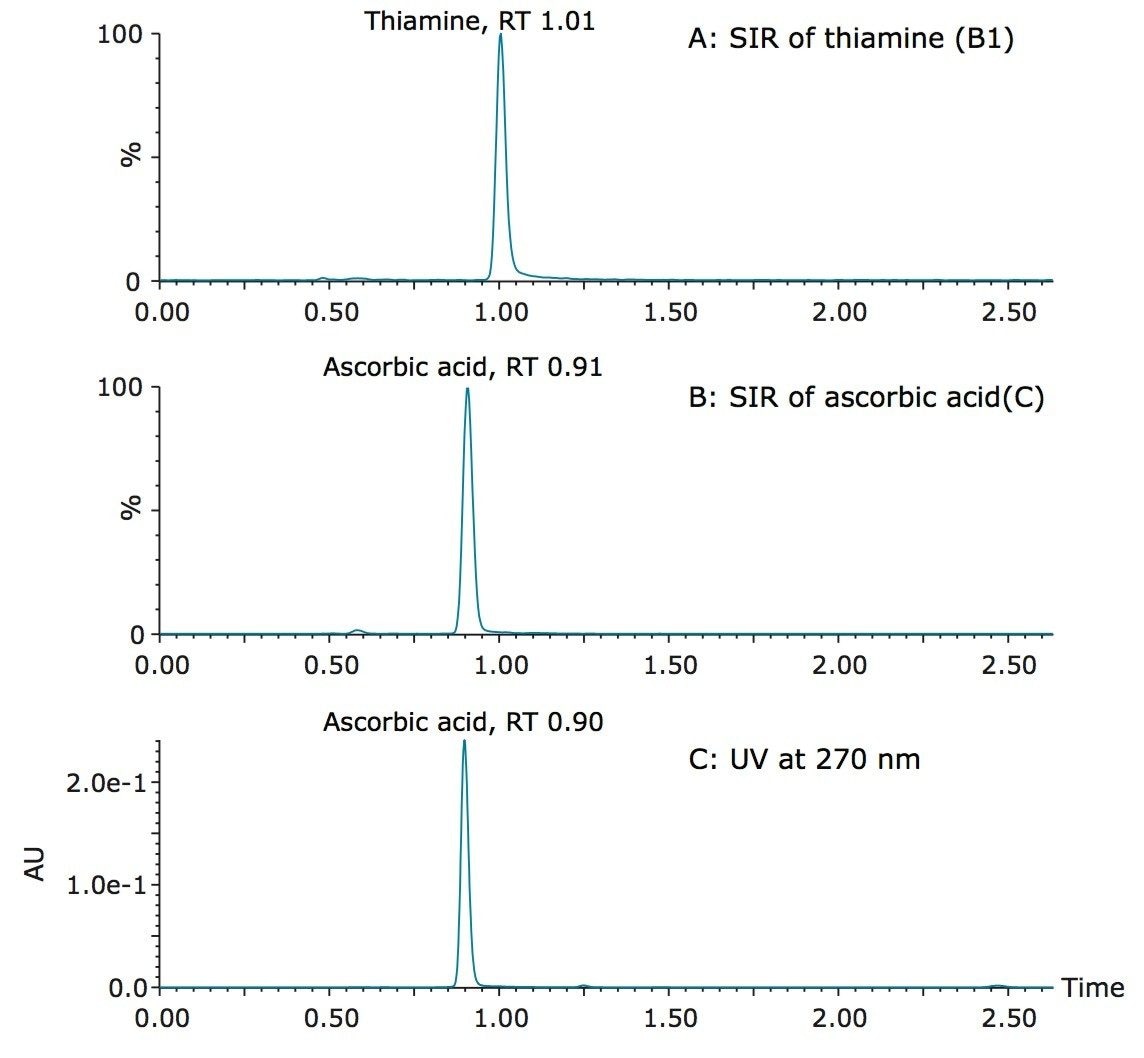
Figure 4 shows the detection of vitamin B5 (calcium pantothenate) in two vitamin water samples. As shown in the UV chromatogram, vitamin B5 could not be detected by UV without additional sample preparation. Vitamin B1 (thiamine) is another vitamin that is difficult to detect using UV. Figure 5 shows an example of the detection of vitamin B1 and vitamin C (ascorbic acid) in a diluted powdered vitamin beverage. Although vitamin C could be detected in the UV chromatogram, vitamin B1 was not detected. Vitamin B1 however, was clearly detected using SIR with the ACQUITY QDa Mass Detector, as shown in Figure 5A.
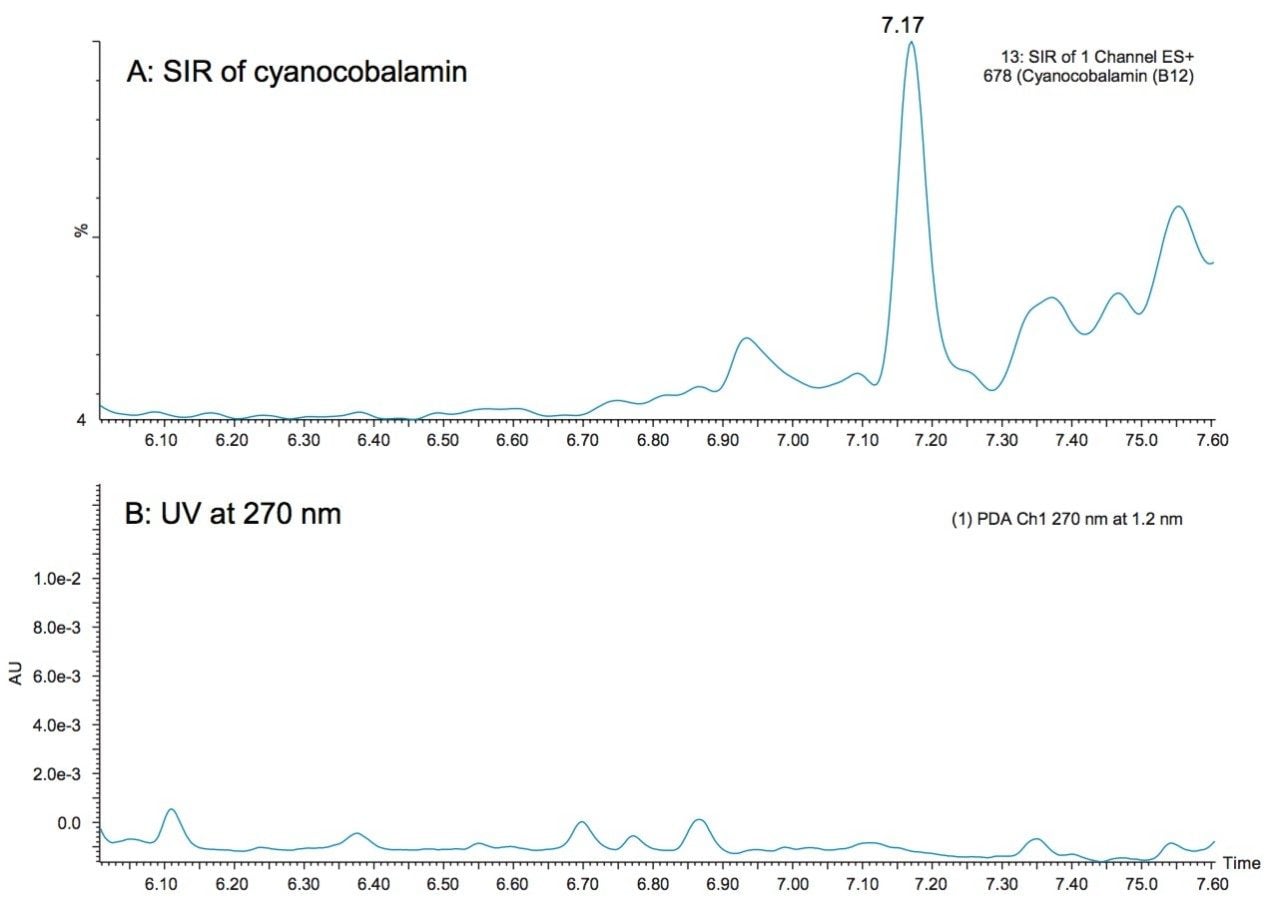
Cyanocobalamin is a WSV that is fortified at very low levels in supplements and foods and it traditionally requires separate methodologies for its quantification. Two-dimensional chromatography is a routine strategy for the detection of this vitamin.4 Figure 6 shows an example of cyanocobalamin detected in the multi-vitamin supplement tablet using the UPLC-MS method presented here. At this level, no peak was apparent in the UV chromatogram (Figure 6B). Mass detection offers the ability to detect vitamin B12 using the same method used to detect vitamins that are fortified at much higher levels. The ACQUITY QDa Detector, which can easily be incorporated into existing LC workflows, offers an easier-to-use method than existing multi-dimensional methods.
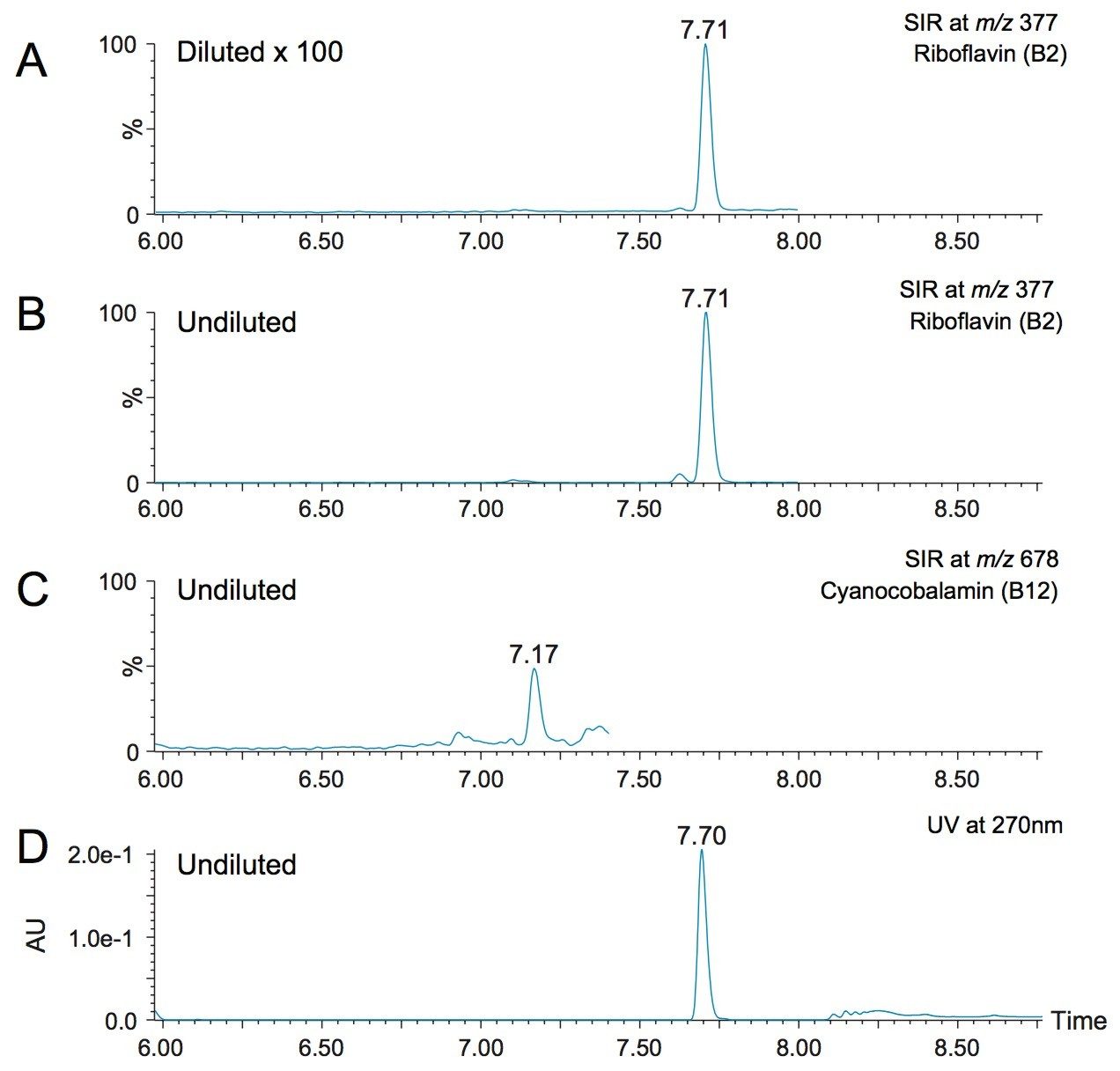
Another challenge that is encountered in vitamin analysis is the wide range of concentrations at which the vitamins are fortified. For the example of the multi-vitamin supplement in tablet form that was used in this study, the label stated that the B vitamins ranged from 6 μg for vitamin B12 (cyanocobalamin) to 16 mg for B3 labeled as niacin (nicotinic acid), with other B vitamins within that range. In this work, the same LC-MS method was used for the analysis of all the vitamins, with different dilution factors of the initial extraction in order to account for the different vitamin levels. Figure 7 shows chromatograms from the analysis of the multi-vitamin tablet. Figures 7A and 7B show the SIR channels of riboflavin (B2) in the 1:100 dilution of the sample and the undiluted sample, respectively. Figure 7C shows the SIR channel of cyanocobalamin (B12) in the undiluted sample. No peak was detected in the diluted sample (data not shown). The UV trace of the undiluted sample at 270 nm is shown in Figure 7D, and the riboflavin peak showed a good response for this sample. The quantified amount for riboflavin and cyanocobalamin were 12.5 ppm and 41 ppb, respectively. These amounts corresponded to 96% and 68% of the label claim of the supplement. Although the label claims were not verified for this work, nor did we undertake a recovery study, this short study demonstrated the feasibility of using the multiple dilution strategy within the calibration range specific in Table 2.
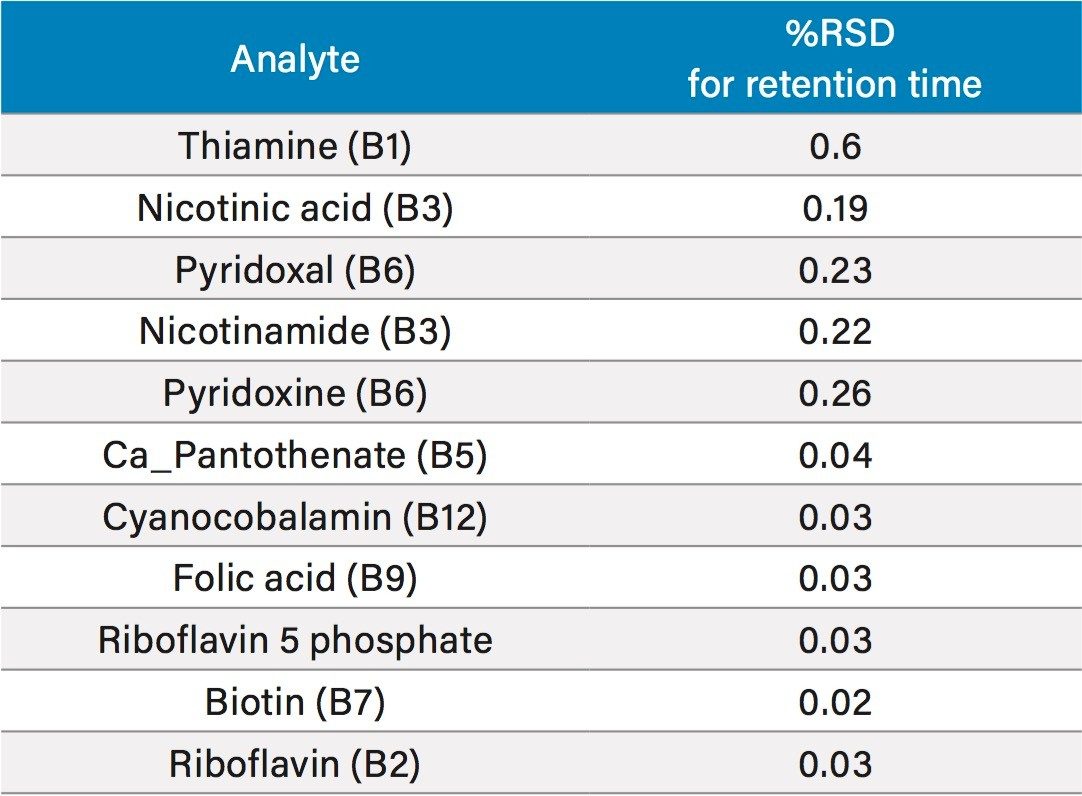
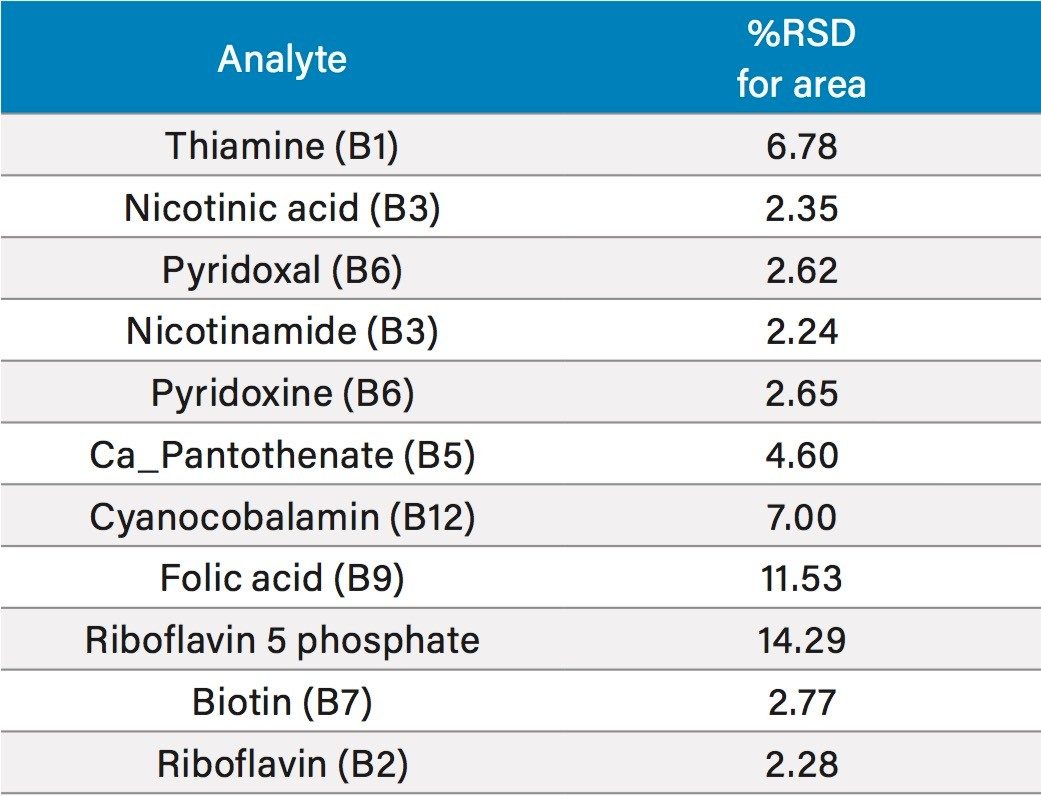
In order to assess the repeatability of the method for the B vitamins, multiple injections at different vitamin levels were assessed. Results for retention time repeatability, and peak area repeatability are shown in Tables 4 and 5, respectively. In Table 4, 10 injections of two different standards had been combined to give a total of 20 injections. Retention time stability was excellent, even for the early eluting water soluble vitamins, with all RSDs at or below 0.6%. Peak area repeatability was assessed with 10 injections at 0.025 mg/L (Table 5). For the majority of vitamins, %RSDs were well below 10%, with the exception of folic acid and riboflavin 5 phosphate, which were the lower responding analytes mentioned above. Vitamin C was excluded from this study as it is known to degrade over time.
This work shows the capability of the ACQUITY QDa Mass Detector to accurately quantify water soluble vitamins at levels that cannot be achieved with UV. The acquisition of SIR channels allows for sensitive and selective quantification of analytes, even when co-elution occurs. This helps to remove the burden of ensuring all analytes are baseline separated and enables the detection of lower levels of vitamins.
The ACQUITY QDa Mass Detector allows new users to:
720004960, November 2016