
Stability screening of excipients in drug products plays a critical role in ensuring drug product quality and safety. The current study successfully demonstrated an efficient UV-based method for the detection and quantitation of oleic acid that is robust as well as sensitive and can be readily deployed in regulated biopharmaceutical environments.
Surfactants such as polyoxyethylene sorbitan monooleate (PS-80) are commonly used in formulated biopharmaceuticals to reduce protein denaturing, aggregation, and surface adsorption to vials and syringes.1 While PS-80 plays a critical role in stabilizing drug products, degradation of surfactants in formulated drugs can decrease overall product efficacy and safety. To this end, characterization, identification, and quantitation of excipients such as PS-80 must be performed to demonstrate the drug product is safe and efficacious in its formulated state. Due to its non-volatile and UV-inactive nature, direct analysis of PS-80 has resulted in methods that incorporate alternative detection techniques such as evaporative light scattering detection (ELSD).2 Yet, given the ubiquitous nature of UV-based instruments in pharmaceutical labs, methods that can utilize existing equipment would be beneficial. Conveniently, the degradation of PS-80 through radical autoxidation or enzymatic processes results in the cleavage of the hydrophobic ester tail (Figure 1) to produce the UV-active fatty acid, oleic acid.1,3,4 Various extraction techniques of fatty acids from biological matrices have resulted in assays that range in degree of sample preparation required, sensitivity, and reproducibility.3-5 UV-based methods that are robust, sensitive, and can be readily deployed in regulated environments are highly valuable in ensuring product quality and safety.
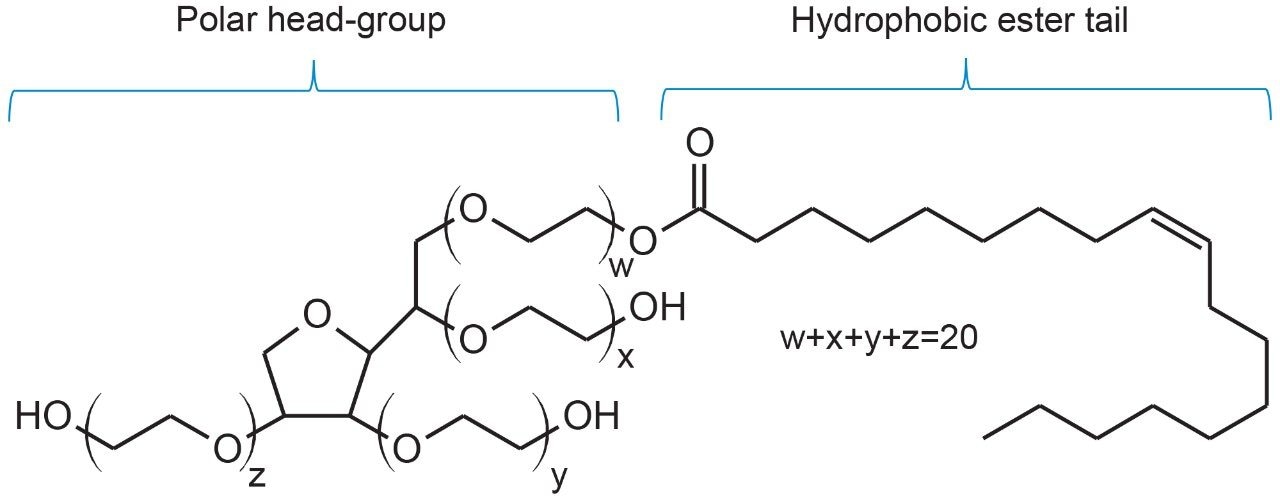
The objective of this study is to demonstrate a robust UV-based method for the determination of oleic acid concentration in drug products that is sensitive and can be performed with minimal sample preparation for straightforward deployment in a laboratory setting.
Polysorbate-80, oleic acid standard, cis-10 nonadecenoic acid standard, and mass spectrometry grade solvents (Optima series) were purchased from Sigma Aldrich. Stock solutions of standards were prepared gravimetrically in acetonitrile. Serial dilutions of oleic acid standards and spiked in samples were prepared using the stock 0.1% solutions. Internal standard cis10-nonadecenoic acid stock was prepared gravimetrically in acetonitrile at a concentration of 0.3%. Formulated samples of infliximab and trastuzumab were used without dilution at a concentration of 20 mg/mL.
|
LC system: |
ACQUITY UPLC H-Class Bio |
|
Detectors: |
ACQUITY UPLC TUV w/titanium flow cell |
|
Absorption wavelength: |
200 nm |
|
Column: |
ACQUITY UPLC Protein BEH C4 300Å, 1.7 µm, 2.1 mm x 100 mm (P/N 186004496) |
|
Column temp.: |
30 °C |
|
Vials: |
TruView LCMS certified 12 x 32mm total recovery vial (P/N 186005669CV) |
|
Sample temp.: |
10 °C |
|
Injection volume: |
1 µL |
|
Mobile phase A: |
H2O, 0.1% FA |
|
Mobile phase B: |
MeCN, 0.1% FA |
|
Isocratic conditions |
|
|
Flow: |
0.200 mL/min |
|
Mobile phase: |
65% B |
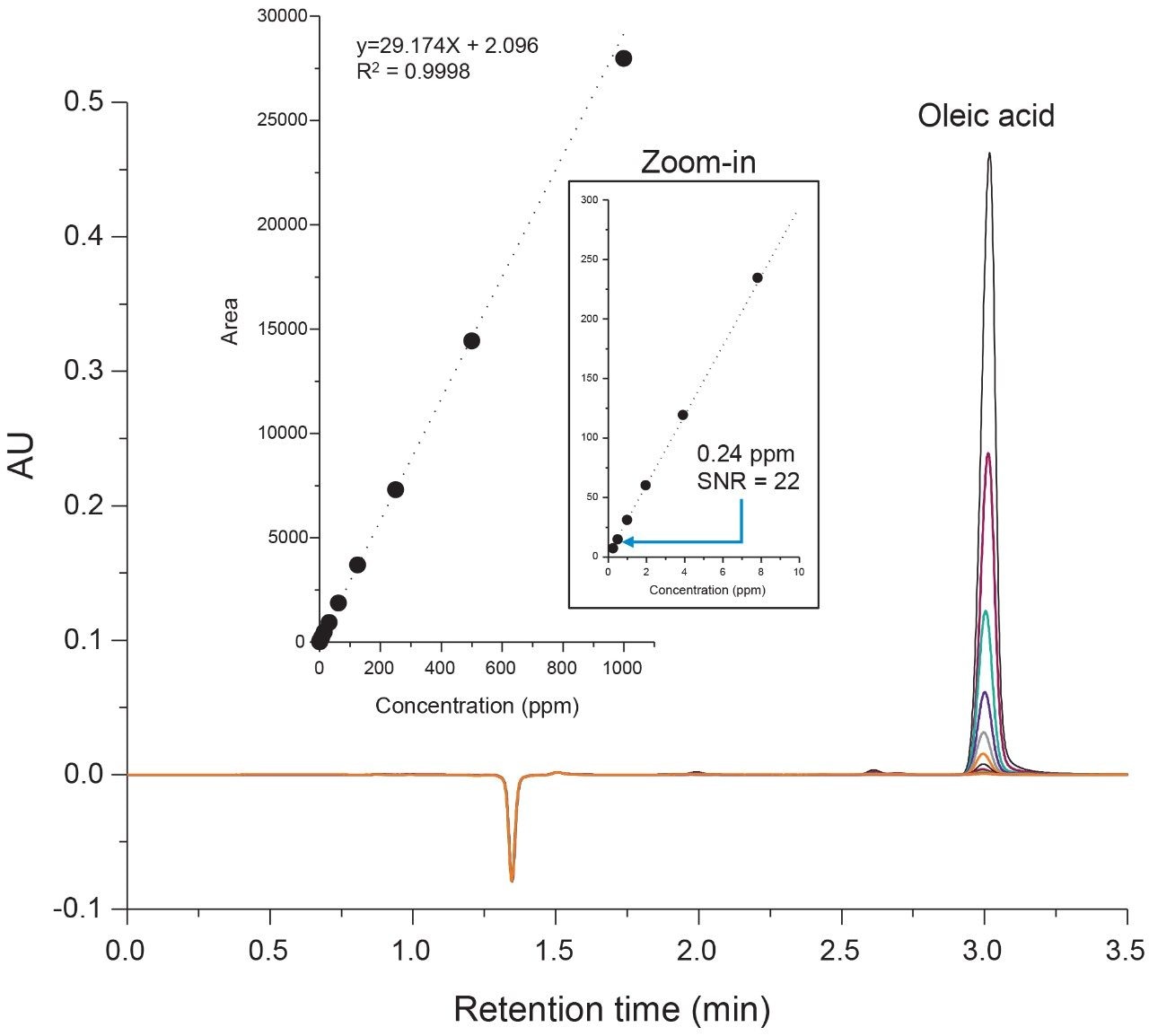
The analysis of fatty acids has been well established in literature over the last few decades using a diverse set of techniques. Legacy methods such as these are quickly becoming outdated as manufacturing processes are modernized as part of a pharmaceutical quality system (ICH Q10). Specifically, HPLC-based separations using wide-bore columns often lack the sensitivity and efficiency needed by today’s standards when considering factors such as post-column dispersion and long run-times and their impact on assay performance.6,7 In light of these factors, some considerations were made in an effort to update the UV-based methodology of the current study. The ACQUITY H-Class Bio System was used as a UPLC platform to minimize system dispersion under isocratic conditions for improved separation performance. In addition, the ACQUITY UPLC Protein BEH C4 Column (300Å, 1.7 µm, 2.1 mm x 100 mm) was used as a lower retentivity column to reduce carryover and increase analysis throughput.
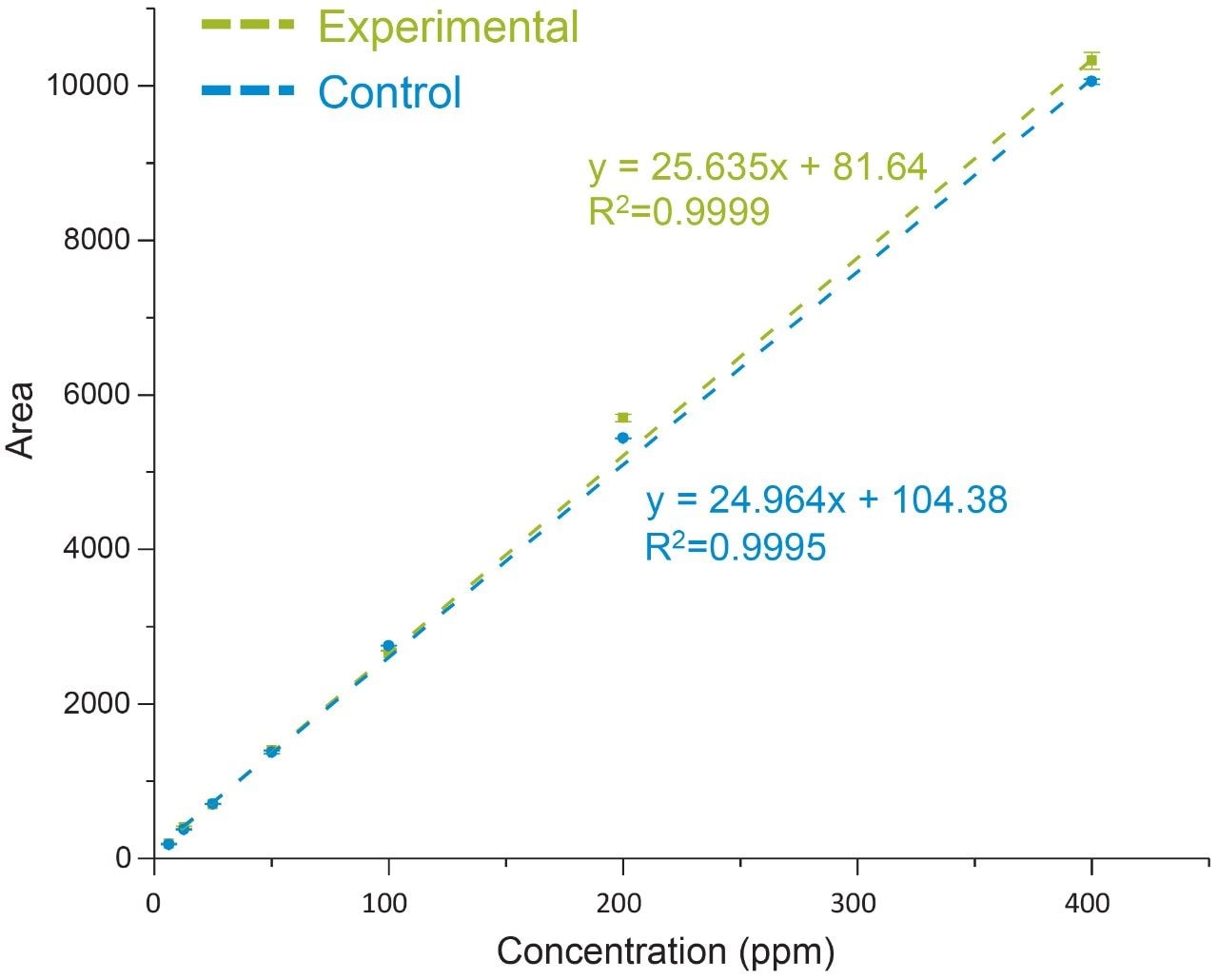
Evaluation of the system and column performance was carried out using a serial dilution series of an oleic acid standard using concentrations ranging from 1000 ppm (0.1%) to 0.24 ppm (0.000024%). Considering the scope of the current study is to isolate and identify one target species, a five-minute isocratic method was used with 65% organic to increase assay robustness. As shown in Figure 2, the lower retentivity of the C4 bonded phase offers the ability to separate highly hydrophobic fatty acids such as oleic acid in under five minutes using conditions that are suitable for both UV and MS-based detection. The high throughput separation enables the ability to maintain good peak shape with minimal dispersion under isocratic conditions. A plot of area versus concentration (Figure 2, inset) showed a high degree of linearity over 3-orders of magnitude with a limit of detection (LOD) of 0.24 ppm, demonstrating UPLC platforms are well suited for UV-based assays that are efficient and sensitive in the detection of oleic acid.
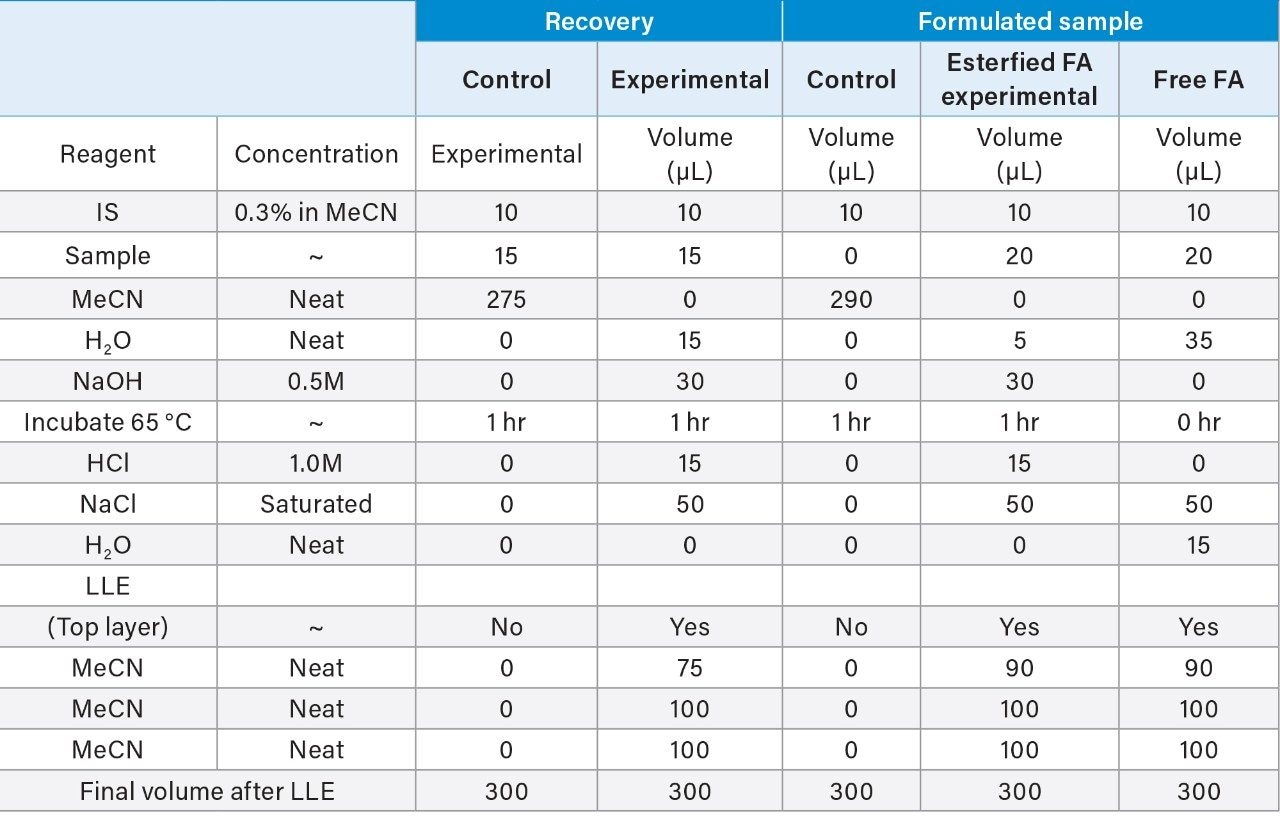
Determination of oleic acid content (free and esterified) has been shown to be used successfully in the assessment and extrapolation of PS-80 degradation and concentration in drug products.2 More recently oleic acid has been used successfully as a predictor for functionality related characteristics such as critical micelle concentration.8 Analyses such as these often employ liquid-liquid extraction (LLE) to isolate fatty acids from sample matrices. However, LLE techniques are not without their own challenges such as matrix effects, extraction volume, and solvent type, which can negatively impact recovery efficiencies and impair accurate quantification. Furthermore, organic extraction solvents are often immiscible with aqueous buffers resulting in laborious dry-down and reconstitution steps. These challenges are addressed in the current study with the development of an extraction protocol that can be used for the extraction of free and esterified fatty acids (after hydrolysis) and is amendable for direct injection onto columns with minimal sample preparation.
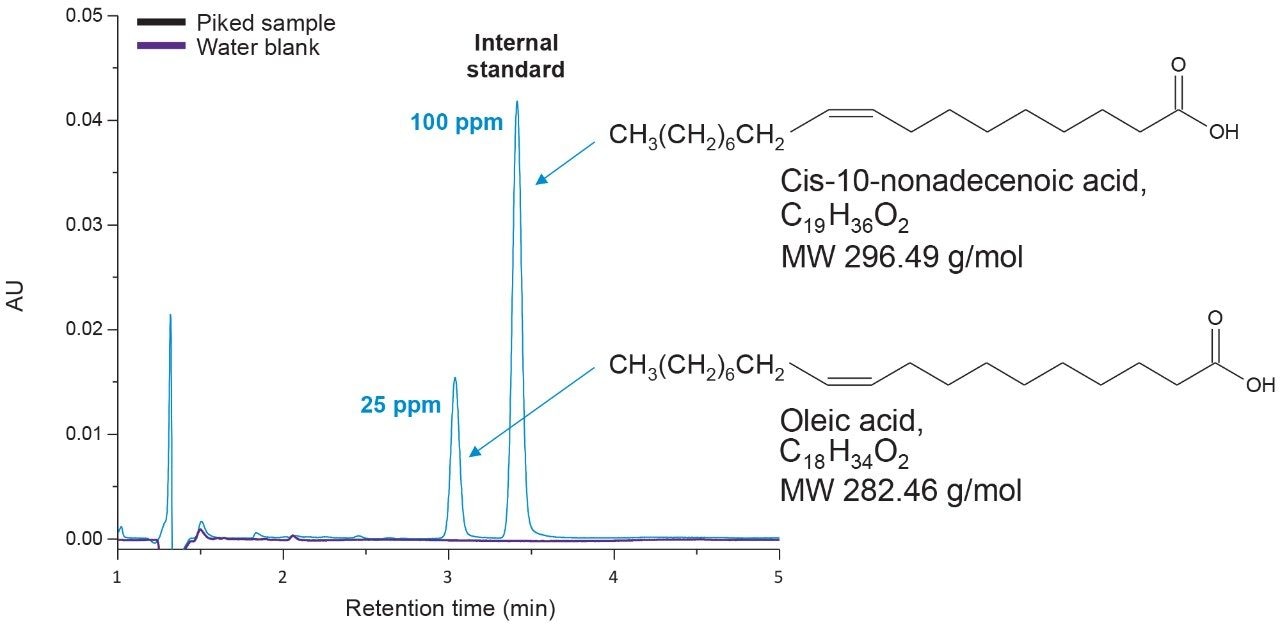
The extraction protocol was evaluated for stability, recovery efficiency, and column performance prior to drug product analysis using an oleic acid standard. Cis-10-nonadecenoic acid was used as an internal standard to correct for non-specific loss of oleic acid during the extraction process. Two sets of oleic acid samples representing experimental and control were prepared as outlined in the extraction protocol (experimental section). Briefly, stock samples of oleic acid were prepared in MeCN at concentrations 0.8%, 0.4%, 0.2%, 0.1%, 0.05%, 0.025%, and 0.0125%. Experimental samples were prepared by taking 15 µL aliquots of each stock and spiking in 10 µL of the IS. NaOH was added to the experimental set and incubated at 65 °C for 1-hr to assess oleic acids stability at the elevated temperature and high pH required for base hydrolysis of PS-80 and drug product biological matrices. The solution was then acidified with HCl to increase solubility of residual biological material in the aqueous phase as well as minimize the column exposure to elevated pH that could decrease column longevity. After acidification, a saturated solution of NaCl was added to induce a phase separation between the acetonitrile and aqueous phase.3 Initial extraction of the top-layer was performed with a reduced aliquot of MeCN to account for the MeCN present in the oleic acid sample and internal standard. A control set was prepared in MeCN to determine recovery efficiency and correct for non-specific sample loss. As shown in Figure 3, a direct injection of 1 µL of the extracted spiked in sample using the same isocratic conditions as before is sufficient to separate oleic acid from the IS with baseline resolution. A water blank (blue trace) performed after the injection showed no observable carry-over on the column. Recovery efficiencies up to 98% were observed for samples containing trace amounts of oleic acid as shown in Table 1 with a marginal decrease in recovery efficiency observed at higher concentrations. Sample loss was determined to be non-specific, as similar trends were observed with the IS as well.
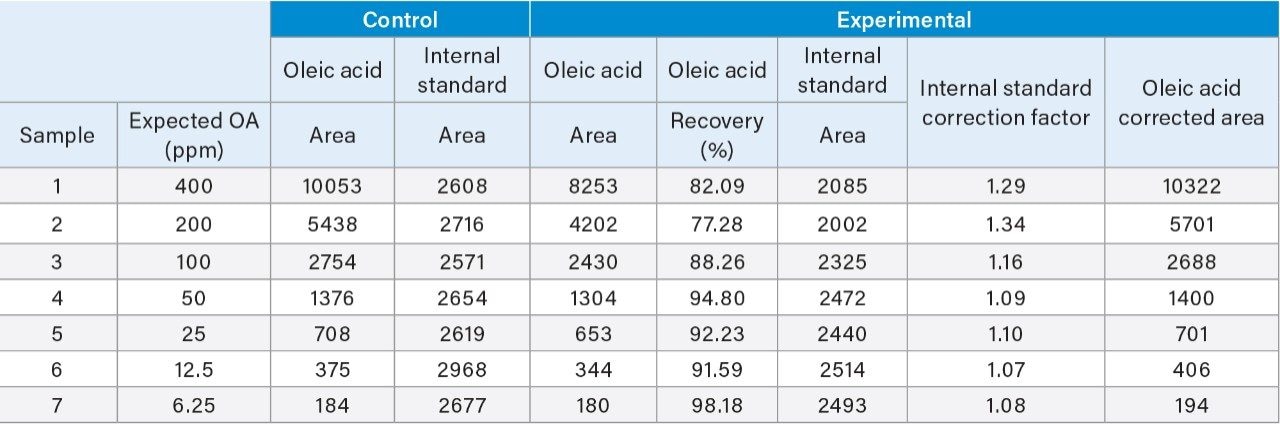
Non-specific loss was corrected for using the average response of the IS in the control sample. As shown in Figure 4, a comparison of the IS corrected experimental and control data showed a high degree of linearity with good agreement between both data sets over the dynamic range of the assay (slope ratio = 1.03). Collectively, these results demonstrate oleic acid and a corresponding IS can be extracted with a high degree of efficiency over a broad working range with conditions that are compatible with direct injection of samples for efficient and accurate analysis of fatty acids.
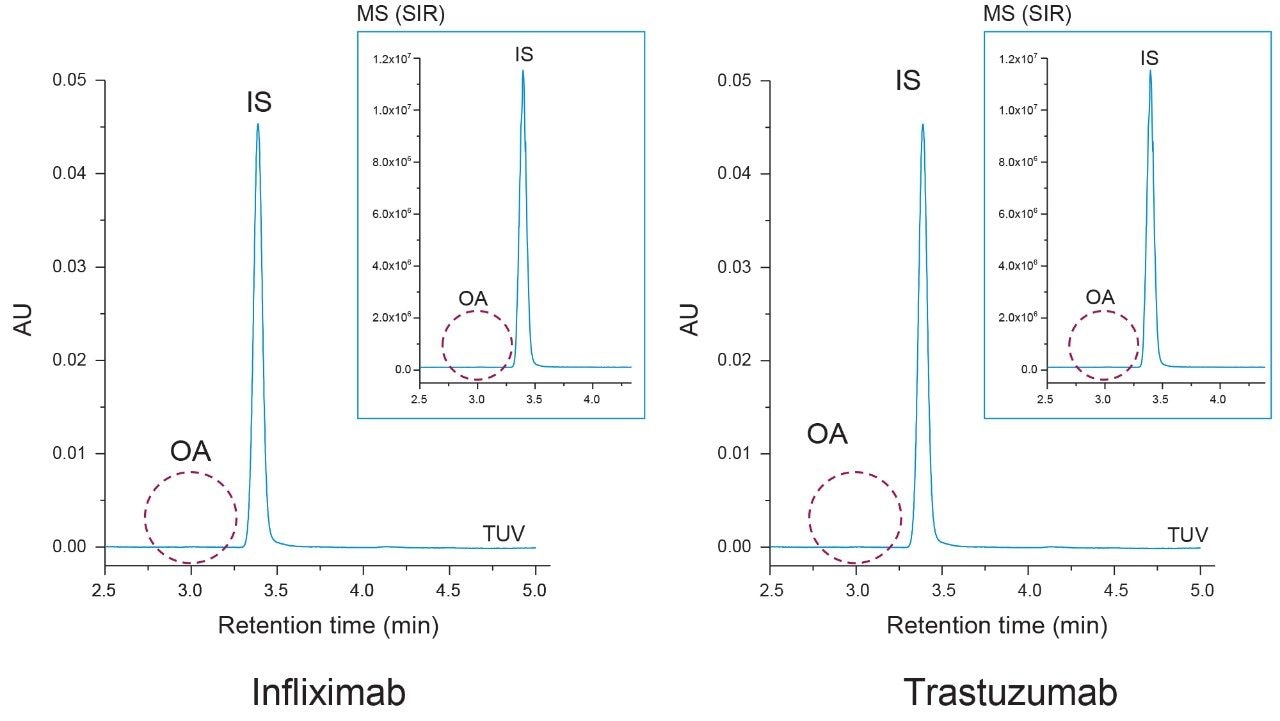
Polyoxyethylene sorbitan monooleate (PS-80) and polyoxyethylene sorbitan monolaurate (PS-20) are two commonly used stabilizing surfactants found in biotherapeutic drug products.1 Representative samples of infliximab (PS-80 containing) and trastuzumab (PS-20 containing) were used to test the applicability and specificity of the proposed method. To determine if free oleic acid was present in the samples as a degradation product, a similar extraction protocol was used as before where the IS was spiked into 400 µg of each sample and the LLE was performed without hydrolysis. Controls were run alongside both samples again to account for non-specific loss.
As shown in Figure 5 oleic acid was not detected in either the infliximab or trastuzumab UV chromatograms. To ensure trace levels of oleic acid were not overlooked an ACQUITY QDa mass detector was placed in-line as an orthogonal detectorfor increased sensitivity. Selected ion recording (SIR) at 283.3 m/z and 297.2 m/z was used to detect oleic acid (OA) and the IS. As shown in the inset of Figure 5 oleic acid was not detected in either sample with the increased sensitivity of the ACQUITY QDa which was previously determined to have a LOD of 60 PPB for oleic acid (data not shown). To determine if esterified oleic acid was present in the form of PS-80, a duplicate sample set with controls was prepared in which the mAb samples underwent base hydrolysis (see Experimental).
As shown in Figure 6, oleic acid was observed to be present in the infliximab sample at less than 1 % relative to the IS (99.08%) peak. The presence of oleic acid in the infliximab sample was confirmed with MS detection (inset). The absence of an oleic acid peak in the hydrolyzed trastuzumab sample highlights the specificity of the assay and confirms that the infliximab sample showed no detectable signs of degradation in the free oleic acid test. These results demonstrate the current method is applicable in the detection of PS-80 degradants in formulated samples.
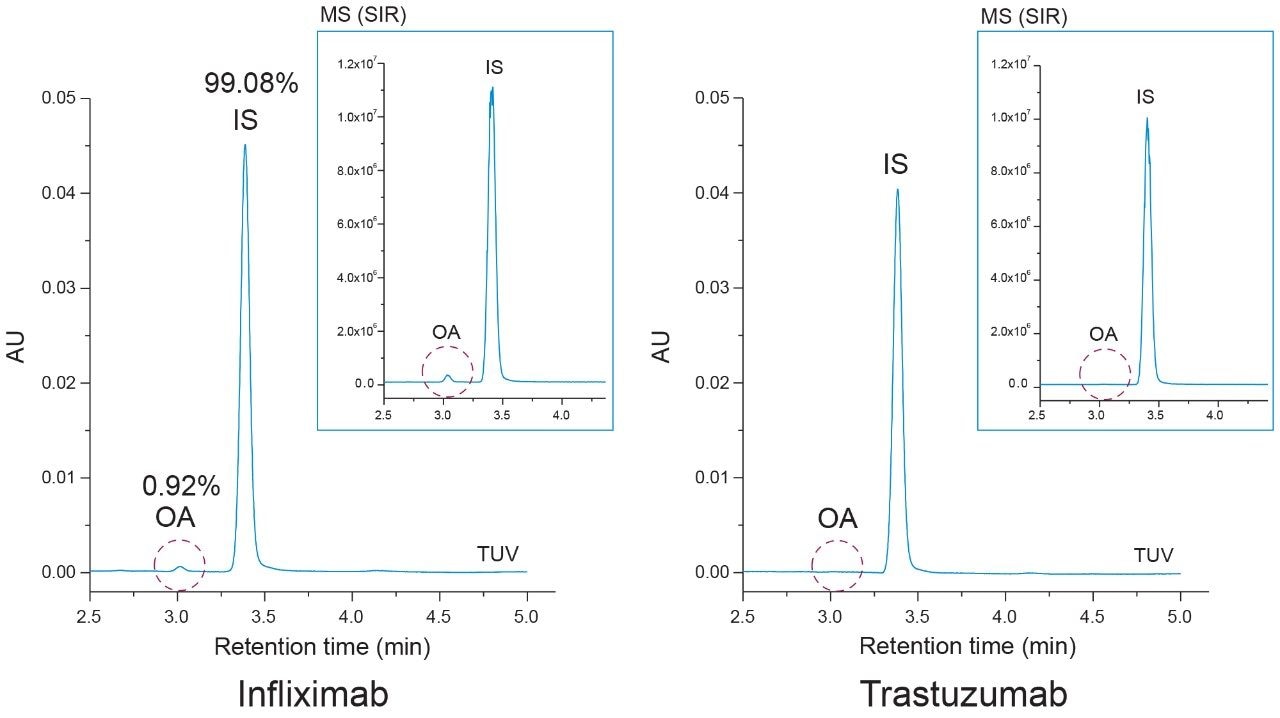
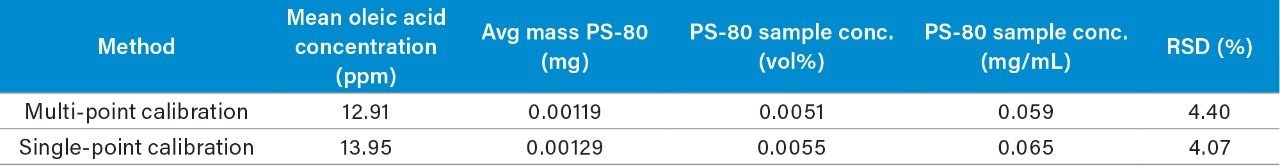
Formulation optimization can benefit from high throughput screening assays for determining conditions that ensure product quality and adequate long-term stability when faced with short timelines. To address this challenge, the current study compared absolute quantification of oleic acid content in the infliximab samples using a multi-point and single-point calibration method. Briefly, the corrected area from the infliximab results was used with the multi-point calibration plot from Figure 2 to determine the oleic acid concentration after accounting for the dilution factor (DF = 15) of the hydrolyzed mAb sample. For the single-point calibration method, the relative peak area of oleic acid (Figure 6) was used to calculate the concentration based on the IS peak area (100 ppm) as well as accounting for the dilution factor. As shown in Table 2, both methods determined the oleic acid content in the hydrolyzed mAb sample to be within 1 ppm of each other with comparable CVs. Furthermore, the oleic acid concentration was used to extrapolate the original concentration of PS-80 in the sample which were determined to be on the same order of magnitude as the product monograph (0.05 mg/mL), assuming the original PS-80 was of high purity. These results suggest a single-point calibration method offers an efficient pathway for the accurate determination of oleic acid concentration in a high throughput manner when compared to a more resource dependent method that relies on multiple calibration points.
Stability screening of excipients in drug products plays a critical role in ensuring drug product quality and safety. Methods that enable accurate assessment of excipients and their related impurities in an efficient manner which can be adapted to current instrumentation are highly valuable in industry. The current study successfully demonstrated an efficient UV-based method for the detection and quantitation of oleic acid that is robust as well as sensitive and can be readily deployed in regulated biopharmaceutical environments.
720006129, February 2018