The ACQUITY UPLC H-Class System configured with the Waters Fraction Manager-Analytical (WFM-A), with its very low system dispersion, exact control of solvent composition, accurate sample injection scheme, and precise fraction collection, allows scientists to perform small scale peptide and impurity isolation with assurance. This application note illustrates the utility of the ACQUITY UPLC H-Class and Waters Fraction Manager-Analytical (WFM-A) systems for the analysis and isolation of a synthetic peptide at the small scale.
Peptides and other biological molecules such as proteins and monoclonal antibodies are becoming increasingly popular in many therapeutic areas such as drug discovery, medical diagnostics, and precision medicine.1,2 Because the initial stages of drug discovery often require only small amounts of target compound, fast and efficient product isolation is a key element to meeting aggressive development timelines. While traditional peptide isolation is generally performed using UV detection, mass-directed isolation makes the purification process easier by providing better differentiation between target peptide and the contaminants formed during synthesis and cleavage. In addition, developing a separation that utilizes both mass and UV detections ensures a more complete chromatographic profile. Compounds that do not ionize, or ionize poorly, will often be detected with low wavelength UV. Conversely, peptides with very low UV extinction will usually be detected with mass analysis. The fluidically-optimized flow path of the UPLC combined with a specially-designed low dispersion fraction collector enable the mass-directed isolation of sharp, narrow product peaks. In this study, we illustrate the utility of the ACQUITY UPLC H-Class and Waters Fraction Manager-Analytical (WFM-A) systems for the analysis and isolation of a synthetic peptide at the small scale. Fast, targeted isolation increases purification efficiency by reducing unnecessary sample handling while generating just enough product for future experiments.
UPLC System controlled by MassLynx Software with FractionLynx Application Manager: ACQUITY UPLC H-Class, ACQUITY Sample Manager, ACQUITY PDA Detector, ACQUITY QDa Detector, ACQUITY Isocratic Solvent Manager (ISM), Waters Fraction Manager-Analytical (WFM-A)
|
Column: |
ACQUITY UPLC Peptide BEH C18, 2.1 × 100 mm, 1.7 μm, 130Å, (p/n: 186003555) |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
0.1% trifluoroacetic acid in water |
|
Mobile phase B: |
0.1% trifluoroacetic acid in acetonitrile |
|
Column temp.: |
30 °C |
|
Makeup solvent: |
9:1 methanol:water, 0.01% formic acid |
|
ISM flow rate: |
0.6 mL/min |
|
Wavelength: |
214 nm |
|
Gradients and injection volumes: as noted in figures |
|
MS scan, 100–1250 m/z, ES+, continuum |
|
|
Sampling frequency: |
2 Hz |
|
Cone voltage: |
10 V |
|
Probe temp.: |
500 °C |
|
ESI capillary: |
Positive mode, 1.5 |

Peptide comprised of 11 amino acids (6 nonpolar, 2 polar, 2 acidic, 1 basic); 1.3 and 5.4 mg/mL, dissolved in water and filtered
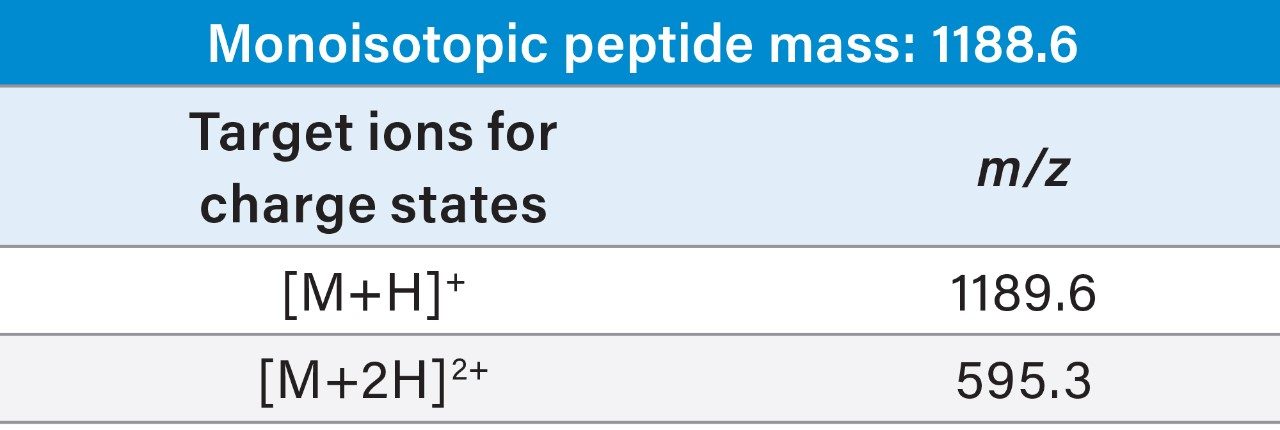
The biopharmaceutical peptide drug candidate, with its inherent complexities, often requires isolation from complicated sample matices. Whether the target peptide is synthesized chemically, or isolated from natural sources, initial experimental studies to determine efficacy in drug discovery may only require small amounts for testing. Undeniably, in some cases, only limited amounts of the potential candidate are available in the sample mixture at the outset of the investigation. The high specificity and sensitivity of mass analysis, combined with the improvements in UPLC and fraction collection technology, ensure the successful implementation of mass-directed purification at the small scale. For the studies described, the ACQUITY UPLC H-Class and Waters Fraction Manager-Analytical systems were employed for the analysis and isolation of a synthetic peptide at the small scale. Expeditious, unambiguous identification and purification of the peptide yielded high purity product with an efficient, streamlined workflow.
In most preparative workflows, crude sample analysis and method development are usually performed to optimize the separation before isolation. The process for small scale mass-directed purification is no different. The ACQUITY UPLC H-Class System configured with the WFM-A can be used with, or without fraction collection, depending upon the objective of the experiment. The selection of a specific fraction method in the sample list turns collection on or off. 3
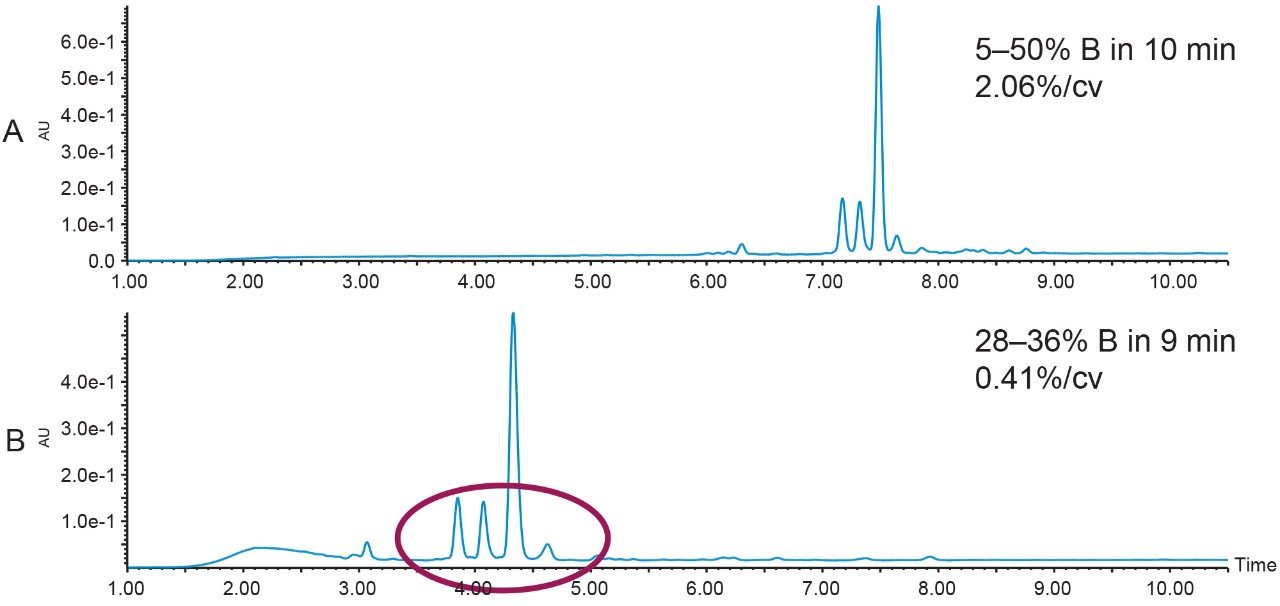
A chromatographic method with a gradient slope of 2.06% change per column volume on a 2.1 × 100 mm reversed-phase column was used to screen the crude peptide. The large peptide product peak and three closely-eluting impurity peaks were present in the initial analysis of the sample mixture. Although this separation was almost baseline-resolved (Figure 2A), focusing the gradient and using a slope of 0.41% change per column volume altered the chromatographic profile and improved the peak-to-peak resolution even further (Figure 2B).
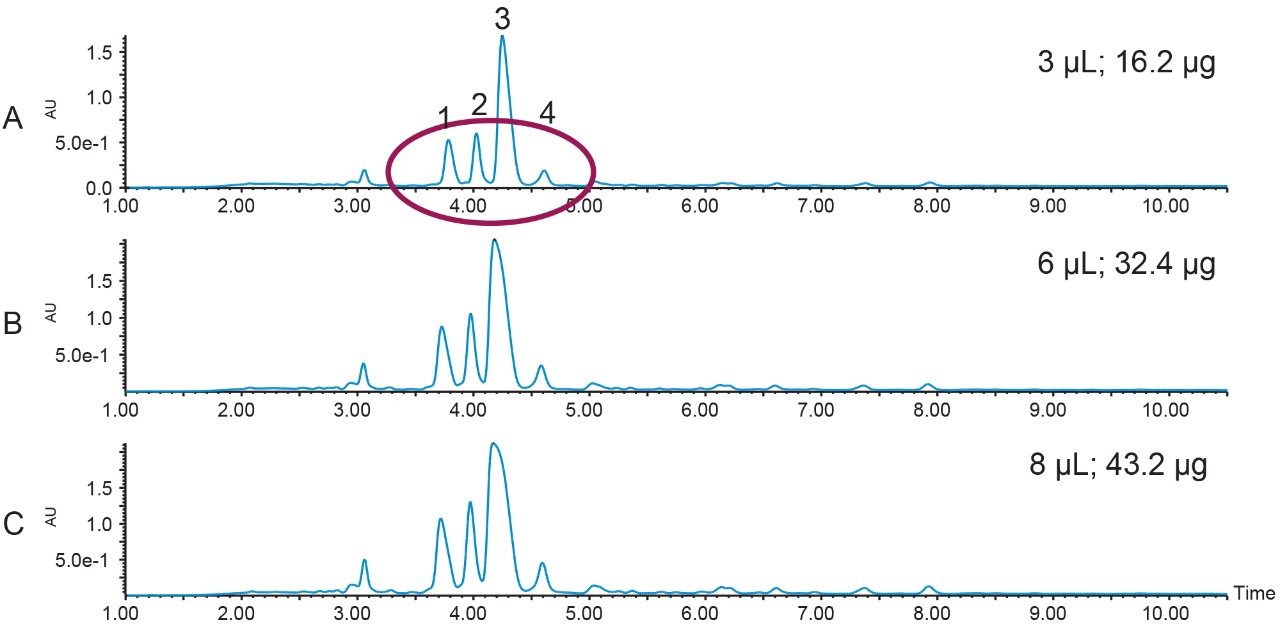
Enhanced resolution between impurities and the target product suggests the possibility of achieving higher product purity values. A loading study was performed to determine the maximum sample load while maintaining the resolution between the desired peptide and its contaminants (Figure 3). While the separation at the higher loadings of 32.4 and 43.2 µg (Figures 3B and 3C) were satisfactory, the more conservative loading at 16.2 µg (Figure 3A) was chosen for prep, since the objective of the isolation was to obtain product with high purity.
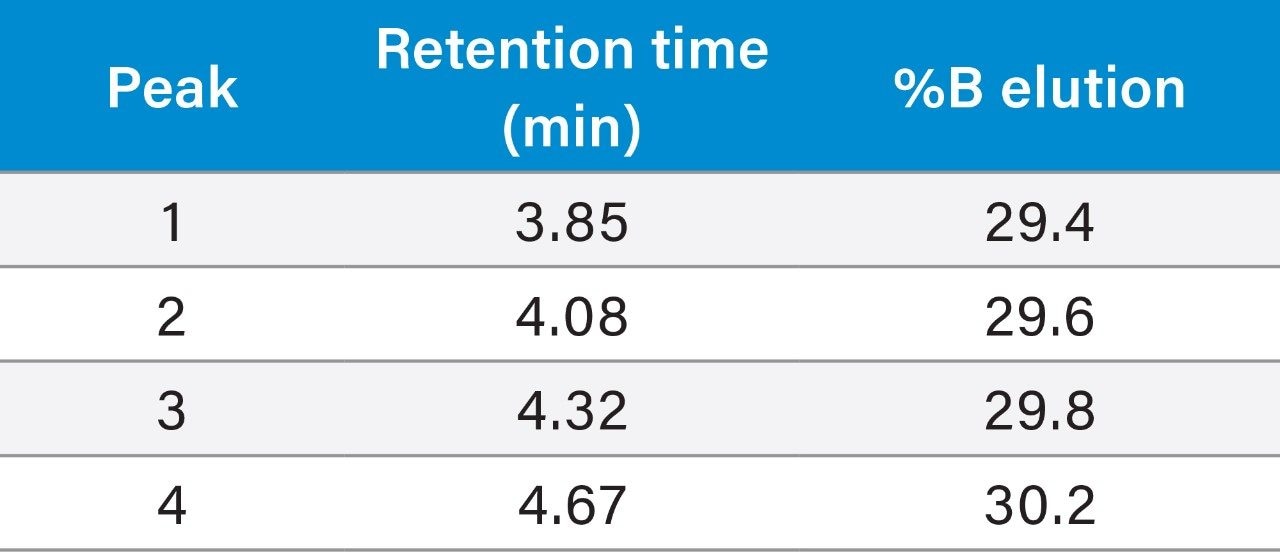
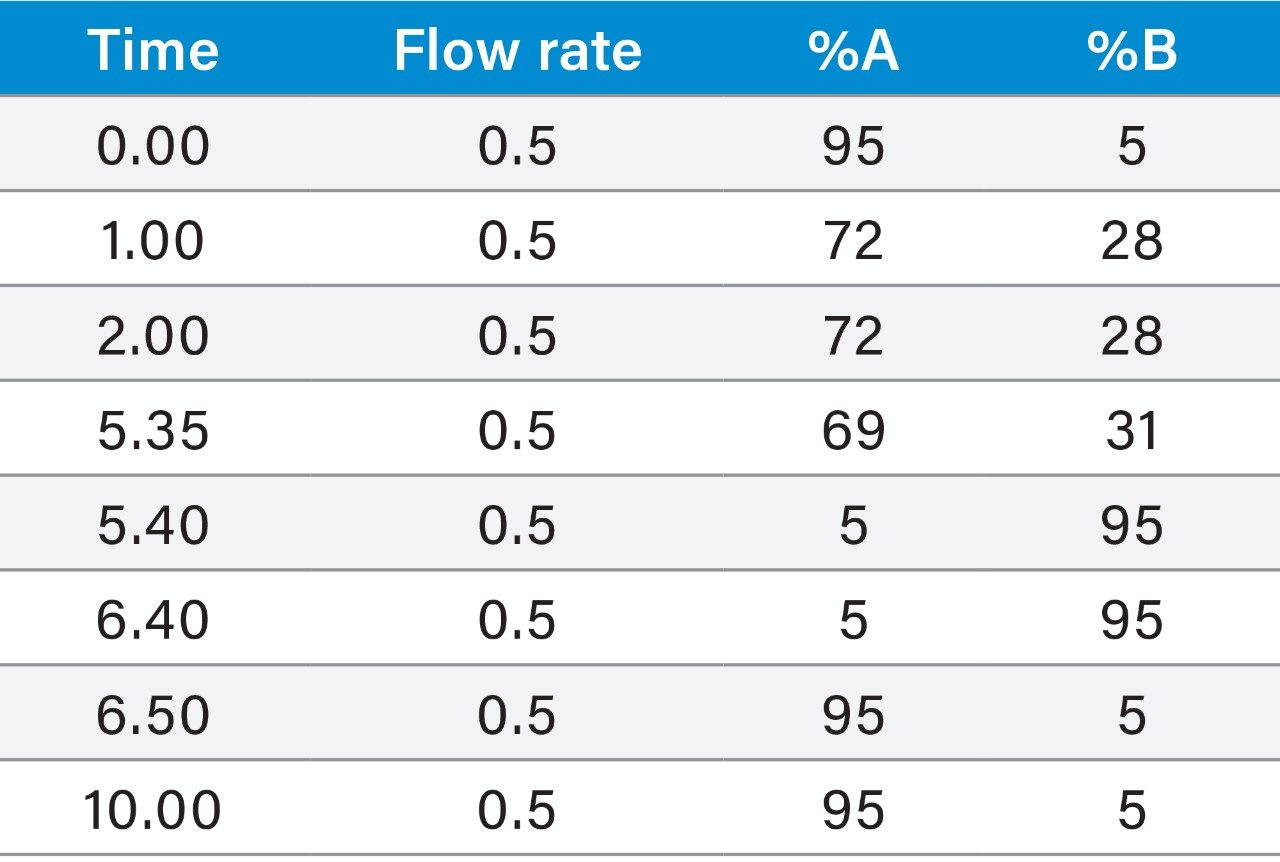
Although the resolution between the peptide and its contaminants was excellent, the total run time of the focused gradient was 15 minutes. Since all of the peaks of interest eluted within the first 5 minutes of the focused gradient, the elution percentage for each of the four peaks of interest in the crude sample chromatogram was calculated (Table 1) and the focused gradient was modified (Table 2) to shorten the total chromatographic run time.
Calculation of peak elution percentage:
System volume estimated at 0.400 mL
Column volume = 0.229 mL
Retention time for impurity 1 = 3.85 min
Offset between the point of gradient formation and the detector = system volume + column volume
Offset = 0.400 mL + 0.229 mL = 0.629 mL
Time for the solvent to reach the detector = Offset/Flowrate
0.629 mL/0.5 mL/min = 1.258 min
Time when the peak elution concentration was formed = Peak retention time – Time to detector – Gradient hold
3.85 min – 1.258 min – 1 min = 1.592 min
Peak elution % = (Time elution concentration was formed/Length gradient segment) × %Change + Initial gradient
(1.592 min/8.94 min) × 8% + 28% = 29.4%
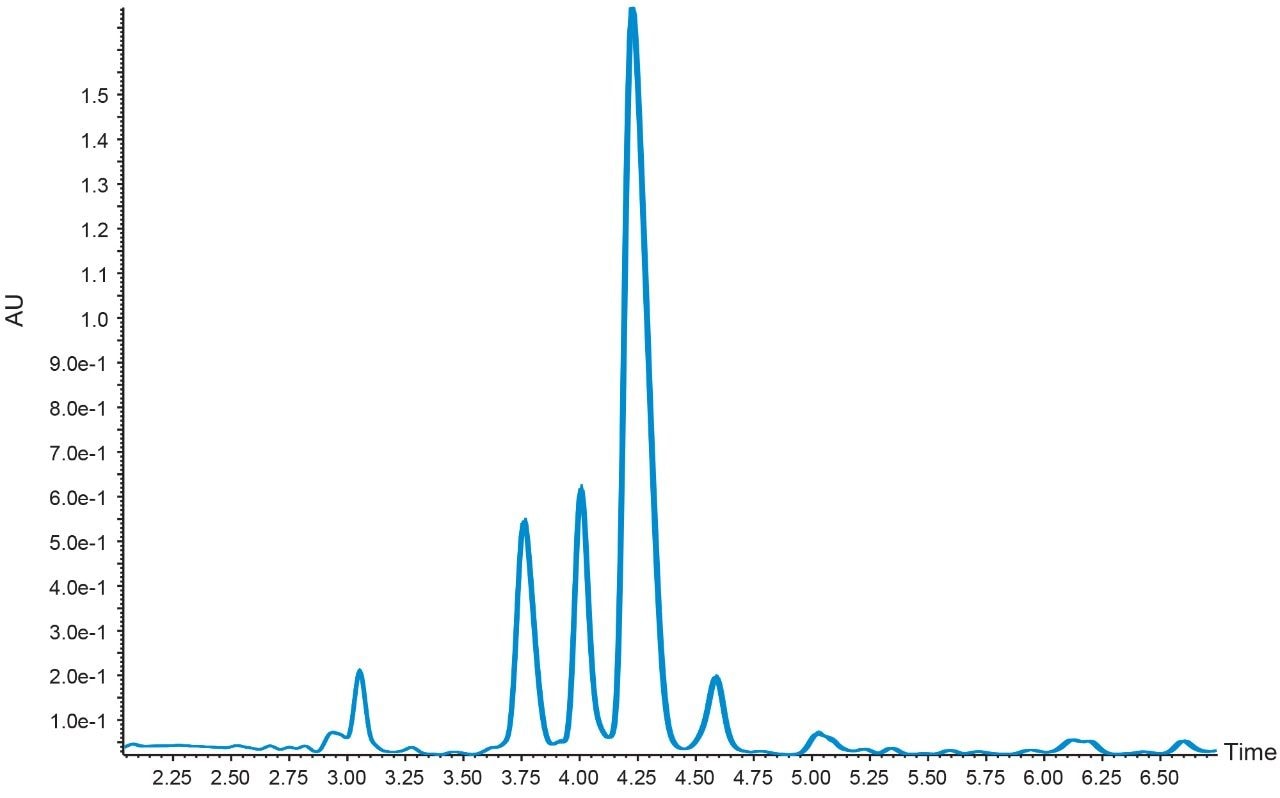
Although the elution percent for the peptide and each related impurity differed by only 0.2%, the resolution was maintained between each of the chromatographic peaks. The ACQUITY UPLC H-Class System, with its low system dispersion, exact control of solvent composition, and accurate sample injection scheme,4-6 provided excellent chromatographic reproducibility, as shown in Figure 4, where ten peptide sample injections overlayed exactly.
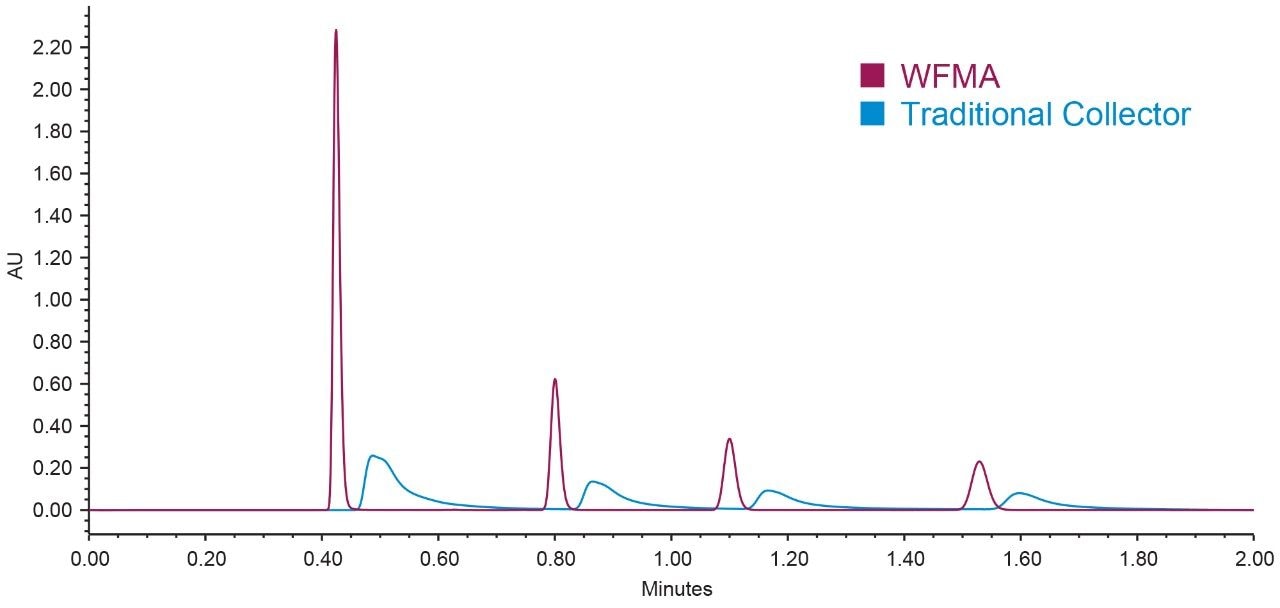
Since small-scale compound isolation can produce collections with very small volumes, the WFM-A was specifically designed to address the challenge of peak dispersion. Figure 5 illustrates the superior peak shape for compounds collected using the low dispersion WFM-A, as compared to the same sample collected using a traditional fraction collector. Fast valve switching, and movement between collection vessels, enables the collection of narrow UPLC peaks. Collectively, these attributes improve the probability of obtaining high recovery with limited sample amounts. Precision control in the UPLC system drives the predictability of the chromatography, as well as the success of the isolation and fraction collection.
The target peptide and three other impurities were isolated by mass-directed purification on the 2.1 × 100 mm column using the modified focused gradient (Table 2, Figure 6).
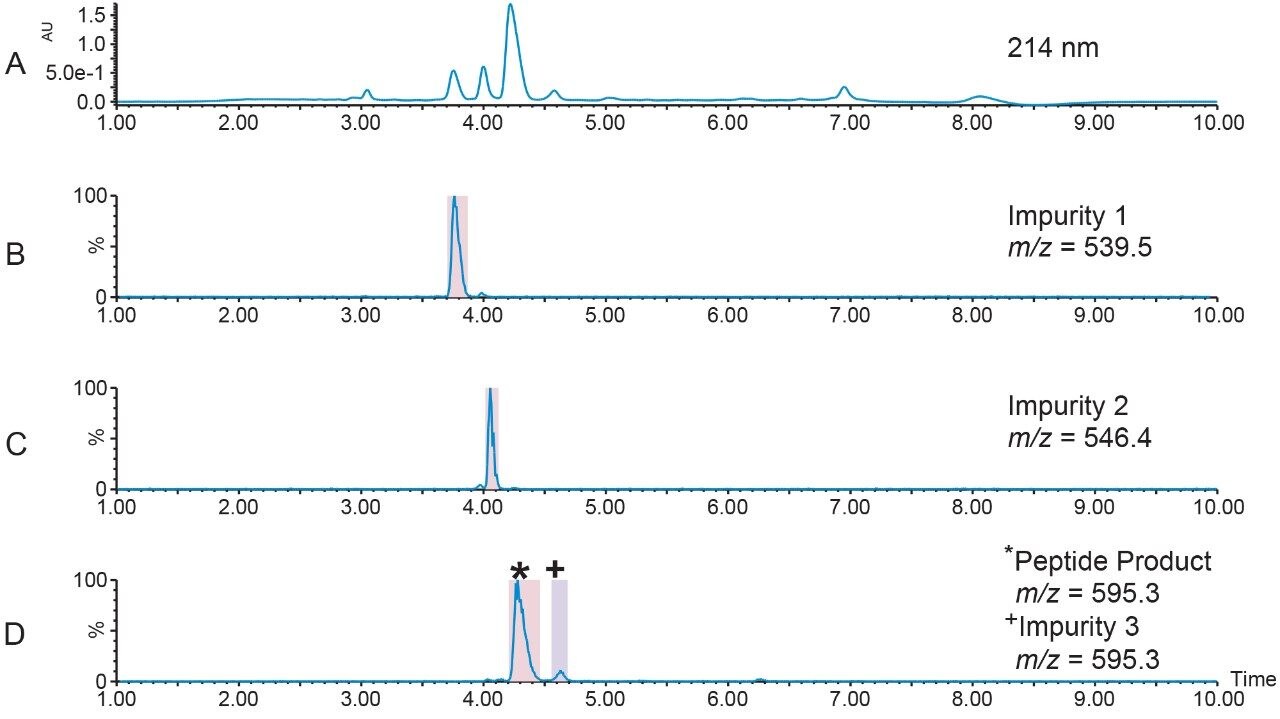
Fraction handling and processing is often challenging because small-scale compound isolation sometimes produces collections with very small volumes. Very narrow UPLC peaks are only a few seconds wide, with some fraction volumes less than 100 µL. Limited fraction volumes containing only modest concentrations of target peak require more injections to ultimately isolate enough material for ensuing studies. Pooling, a feature available in FractionLynx Software, simplifies fraction handling by increasing the total volume of the collected product. Figure 7 shows the fraction collection and pooling for a set of three injections. Peak 1 from each injection was collected into tube 1, peak 2 from each injection was collected into tube 2, and so on. Pooling also increases the number of injections for the defined fraction collection plate. Unattended system operation also streamlines the process and improves efficiency.
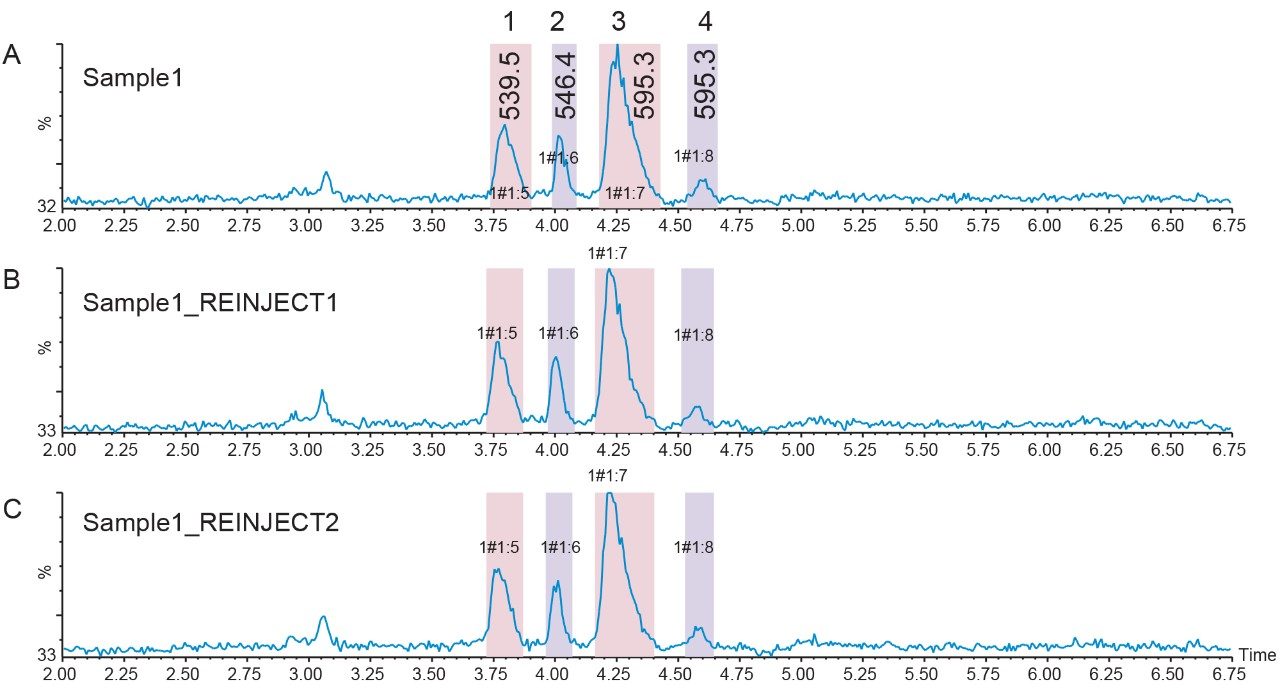
Forty-five small scale peptide and impurity isolations were pooled by peak mass and analyzed with the original screening gradient. The first two eluting impurity peaks (m/z 539.5 and m/z 546.4), as well as the peptide product peak, were very pure. A co-eluting contaminant decreased the purity of the third impurity peak, which eluted after the peptide (Figure 8).
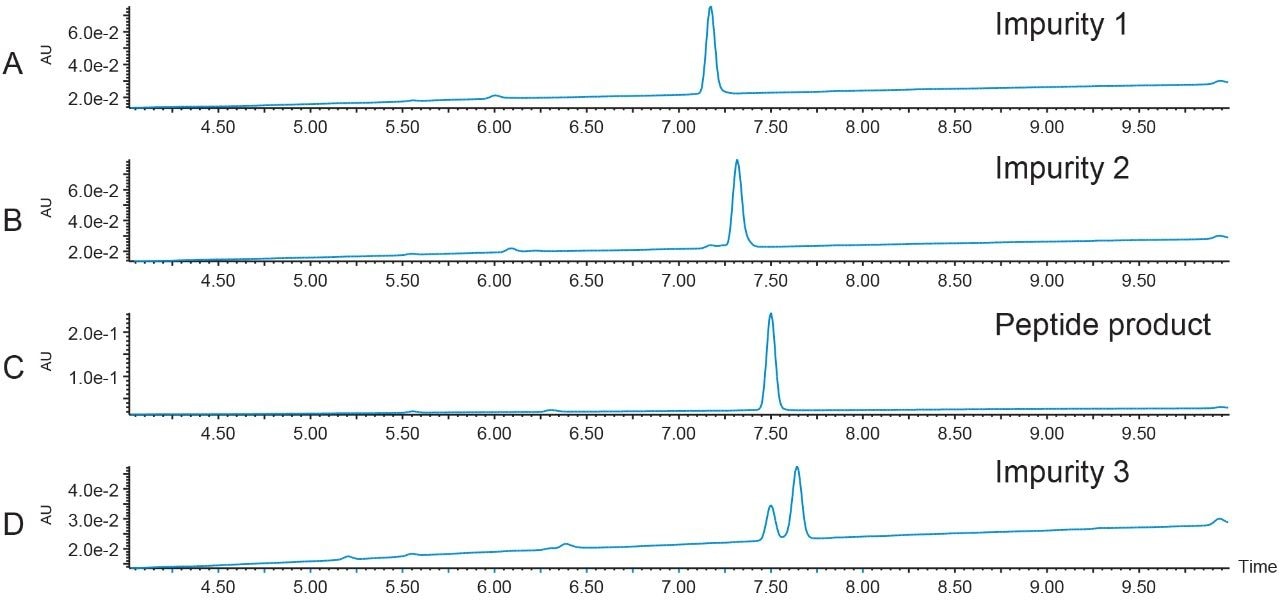
Peptide recovery was calculated at 98.5%, based on the number of area counts in one injection of the pure fraction compared to the total number of area counts for all 45 injections in the total pool volume.
Area counts for one 8 µL injection from the fraction pool = 12551
Total area counts in the fraction pool = 8684039
Total fraction pool volume = 5617 µL
8684039/5617 µL = 1546 counts/µL × 8 µL = 12368 counts
12368 counts/12551 counts × 100 = 98.5% Peptide recovery
Small scale mass-directed peptide isolation with fraction pooling reduces sample handling, which saves time and generates just enough product for additional analysis or future experiments.
The ACQUITY UPLC H-Class System configured with the Waters Fraction Manager-Analytical (WFM-A), with its very low system dispersion, exact control of solvent composition, accurate sample injection scheme, and precise fraction collection, allows scientists to perform small scale peptide and impurity isolation with assurance.
Fast valve switching and movement between vessels, as well as a fraction divert valve with very low dispersion volume, facilitates narrow target peak collection with high recovery and increases confidence in compound isolation.
Small scale peptide isolation saves sample, time, and resources, resulting in improved efficiency in the purification process.
720006391, October 2018