
For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
The COVID-19 pandemic has resulted in the development of mass spectrometry based methods to characterize, identify, and quantify proteins. These methods are aimed at understanding the structural biology and interaction mechanisms of SARS-CoV-2, or as a complementary method to detect relevant markers. Targeted mass spectrometry, through the detection of viral peptides in proteolytically digested body fluids, has been suggested as a SARS-CoV-2 detection method.1 The work presented here demonstrates application of the MassLynx Skyline Interface for automated peptide Multiple Reaction Monitoring selection and optimization with a Xevo TQ-XS Tandem Quadrupole Mass Spectrometer.
COVID-19 is an ongoing global pandemic caused by the SARS-CoV-2 virus. Efforts to overcome limitations in the current standard polymerase chain reaction (PCR) diagnostic testing capacity and associated reagent shortages have driven the quest for new diagnostics.2,3 The SARS-CoV-2 virion is unusually protein rich, with Spike glycoprotein (SPIKE) and Nucleoprotein (NCAP) accounting for the majority of the protein complement. SPIKE recognizes human angiotensin-converting enzyme 2 in the initial stage of infection. NCAP is a structural component of the viral particle involved in replication and transcription of the genome.4 The detection and quantification of SARS-CoV-2 proteins by a targeted LC-MS method is therefore being considered as an alternative method for COVID-19 viral load determination. As a result, LC-MS methods are currently under development as part of a community-based effort to develop a ‘A Universally Adoptable Corona Multiple Reaction Monitoring Assay’.5 Here, we have applied several complementary approaches for selecting and identifying surrogate peptides, including Multiple Reaction Monitoring (MRM) transitions, to detect and quantify SARS-CoV-2 proteins.
Tryptic-Lys C peptides from a combined digestion procedure of recombinant SARS-CoV-2 SPIKE and NCAP proteins, as individual standards and spiked in Universal Transport Medium (UTM) matrix, respectively, were obtained in freeze-dried form from Cov-MS.5 The resulting peptides were analyzed in MRM mode of analysis using an ACQUITY UPLC I-Class PLUS System interfaced to a Xevo TQ-XS Tandem Quadrupole Mass Spectrometer. Quantitative data analysis was conducted with TargetLynx and Skyline.6
|
LC system: |
ACQUITY UPLC I-Class PLUS |
|
Vials: |
QuanRecovery Vials with MaxPeak HPS |
|
Column(s): |
ACQUITY PREMIER Peptide BEH C18 300 Å, 2.1 mm x 50 mm, 1.7 µm |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
5 µL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
0.1% formic acid in H2O |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
MS system: |
Xevo TQ-XS |
|
Ionization mode: |
ESI positive |
|
Acquisition mode: |
MRM |
|
Capillary voltage: |
0.5 kV |
|
Collision energy: |
peptide/transition optimized |
|
Cone voltage: |
35 V |
|
Time (min) |
%B solvent |
|---|---|
|
0.0 |
5 |
|
5.5 |
33 |
|
5.6 |
85 |
|
7.0 |
85 |
|
7.1 |
5 |
|
8.0 |
5 |
|
Software |
MassLynx TargetLynx MassLynx Skyline Interface Skyline |
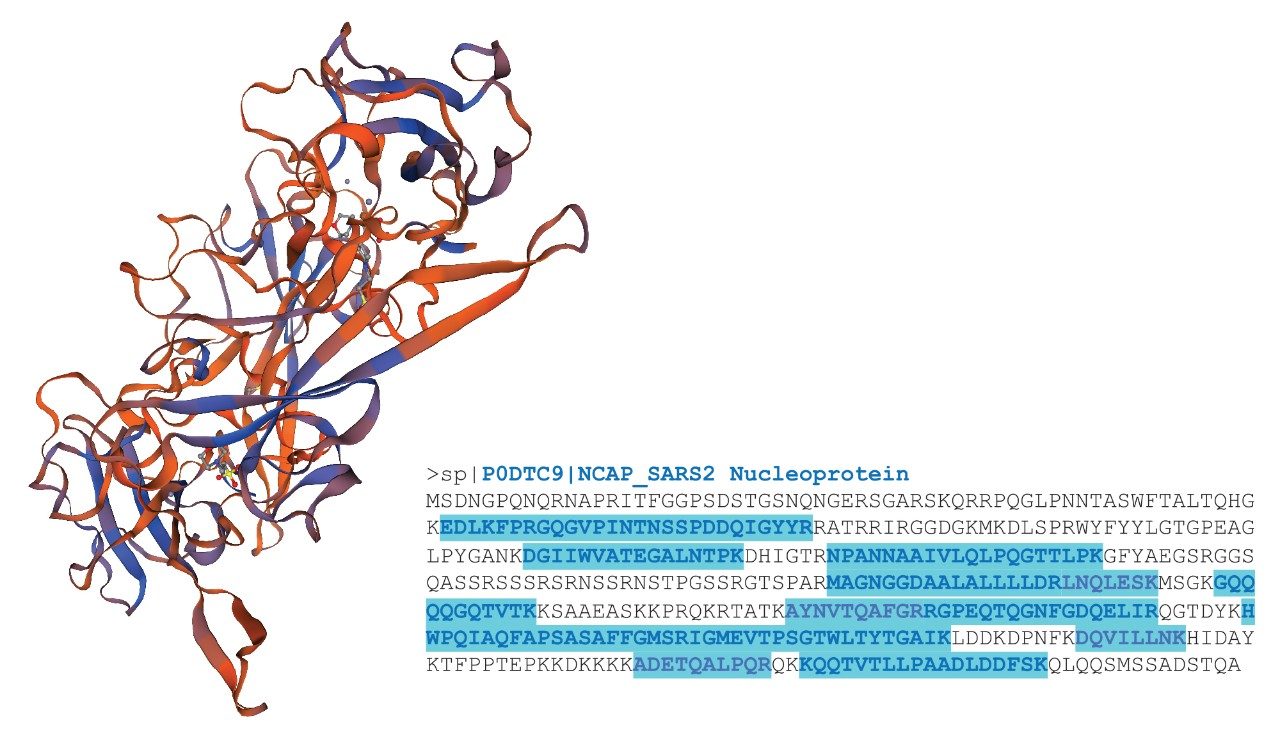
The NCAP amino acid sequence and coverage, as defined in the original Cov-MS standard operation procedure (SOP), is shown in Figure 1. Together with the primary amino acid sequence of the SPIKE protein, these form the basis for the MRM selection and optimization process. The MassLynx Skyline Interface (MSI) process was followed for automated optimization and fine tuning of a tandem quadrupole MRM method,7 as well as the detection of any additional candidate signature peptides. In short, MSI conducts an automated four-step process whereby first the retention time of the peptides is determined. Next, the most sensitive precursor/product ion pairs (MRM transitions) for every peptide are determined using a default calculated collision induced dissociation (CID) fragmentation energy. This is followed by optimization of the individual transition collision energies. Finally, an MRM method with appropriate acquisition windows is created. As shown on the left hand side of Figure 2, the appropriate peptide and transition settings are specified within a Skyline document. Next, as shown on the right hand side of Figure 2, the document is specified in MSI alongside with other methods files (tune page, acquisition method, LC gradient), sample position and injection volume.
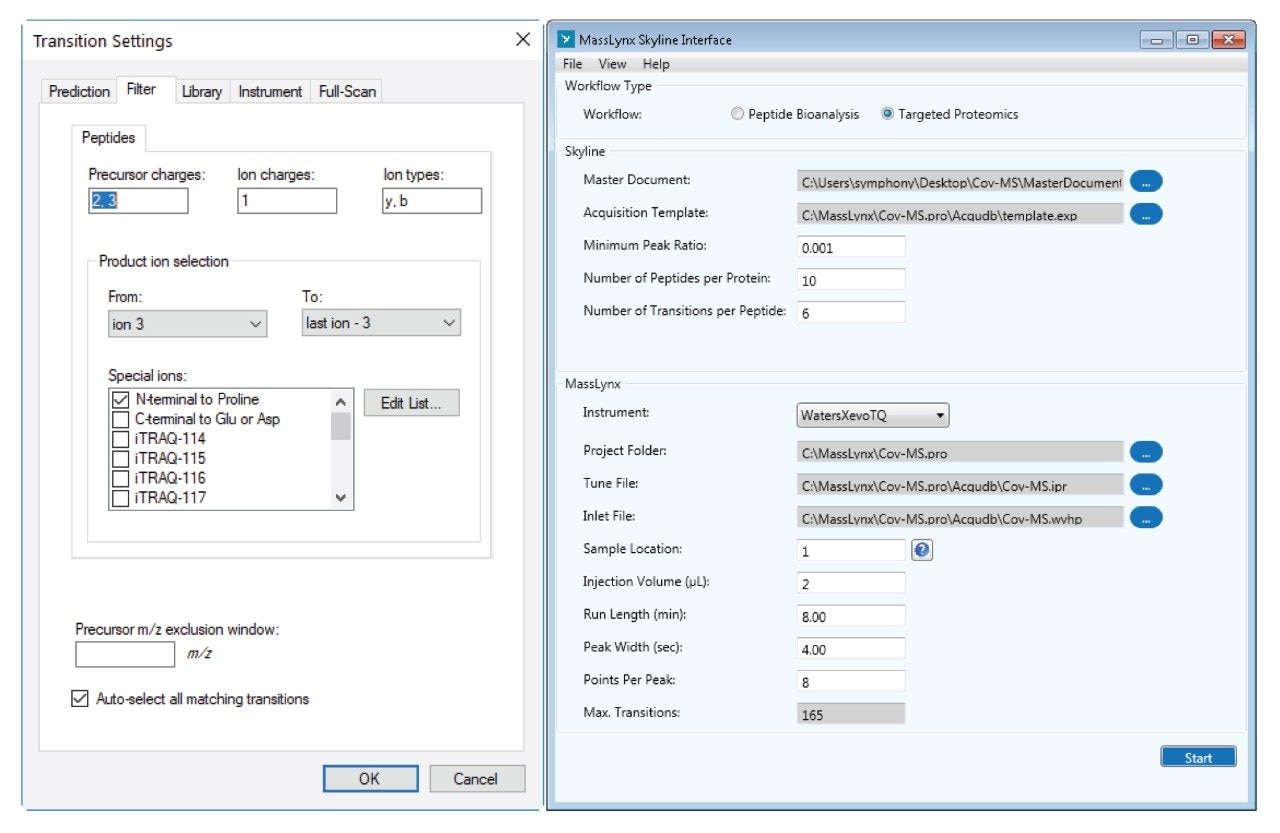
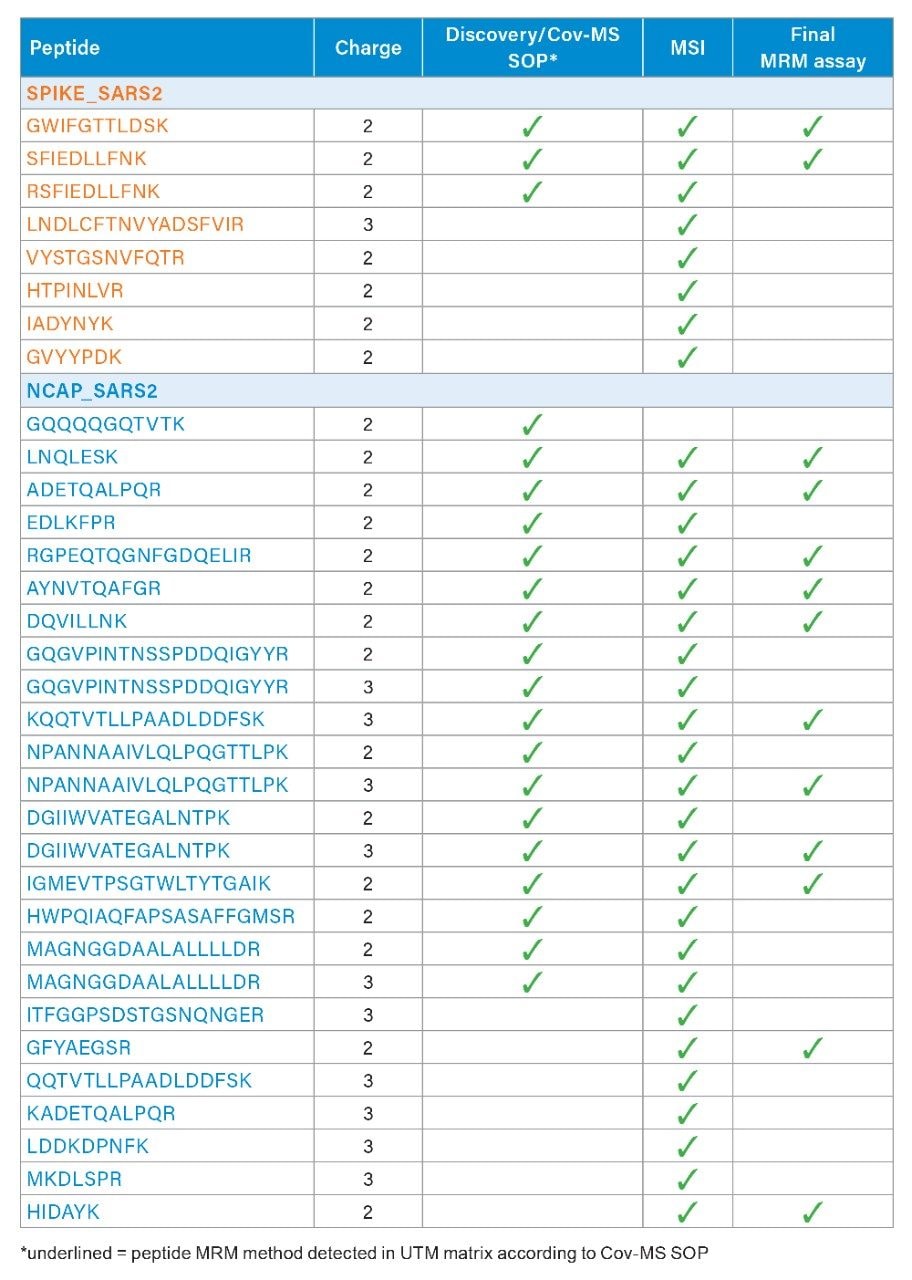
The NCAP and SPIKE proteins were in-silico digested allowing for one sequence motif specific missed cleavage to optimize the MRM transition settings for the discovery identified peptides and potentially identify any additional relevant peptides. At this stage of the optimization process, the number of transitions per peptide was set at six to retain flexibility in transition selection during downstream acquisition and quantification experiments. The detected peptides are overviewed in Table 1. The result summarised in Table 1 overview (1) the peptides specified in the original Cov-MS SOP, identified using a discovery-based data-independent analysis (DIA) method and validated by means of MRM, (2) the SARS-CoV-2 peptides identified by the MSI process, and (3) the peptides that were retained in the final MRM method based on LC-MS response and suitability/specificity.
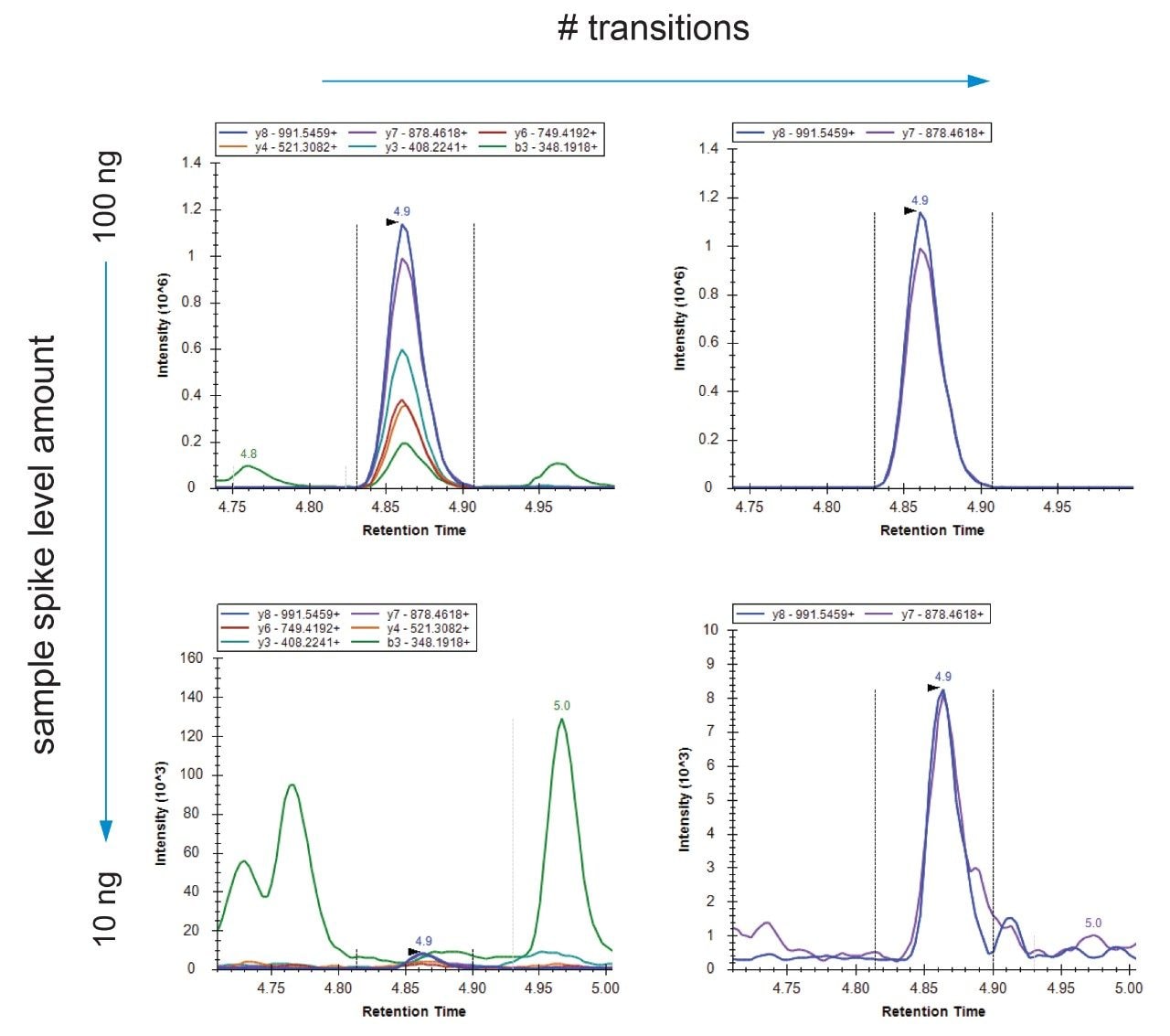
To illustrate the importance of using the correct number of transitions/fragment ions for quantitation, MRM chromatograms for one of the NCAP peptides are shown in Figure 3. The two top chromatograms illustrate the MRM acquisitions using the maximum and two most abundant transitions per peptide, respectively, for the second highest spike level (100 ng) as specified in the original Cov-MS SOP. The maximum number of transition experiment provides the highest flexibility in terms of selecting, post-acquisition, the least interfered MRM transitions, which could be matrix and patient sample dependent. However, using the two most abundant transitions provides the best tandem quadrupole duty cycle and potentially also the best signal-to-noise. The latter is demonstrated by the two bottom chromatograms shown in Figure 3, representing the second lowest detected spike level (10 ng) of the dilution series, illustrating an interference when using the maximum number of transitions per peptide, thereby hampering detection, leading ultimately to decreased performance in terms of achievable lower limits of detection (LLOD) compared to the use of the two most abundant and non-interfered MRM transitions.
The efficient and accurate development of MRM LC-MS-based method, as discussed in this clinical research brief, requires reliable and automated processes to complement prior peptide selection rules based on expert knowledge criteria, discovery-based identification results, or information available in public repositories. To meet the challenge associated with the novel coronavirus, we have applied an easy-to-use and streamlined MRM selection and optimization process, as incorporated in MSI. The obtained results were successfully applied and tested on a Xevo TQ-XS Tandem Quadrupole Mass Spectrometer. Moreover, the obtained MRM methods can be readily transferred to other LC-MS systems for further optimization and application of the MRM method to detect SARS-CoV-2 proteins.
The Cov-MS consortium is kindly acknowledged for making evaluation kits available as part of a community-based effort to design a SARS-Cov-2 MRM method.
Laurence Van Oudenhove, Nikunj Tanna, Jan Claereboudt and Hans Vissers (Waters Corporation); Bart Van Puyvelde, Simon Daled, Dieter Deforce and Maarten Dhaenens (Pharmaceutical Biotechnology, University of Ghent); Katleen Van Uytfanghe (Department of Bioanalysis, University of Ghent).
720006967, August 2020