Vitamins are minor constituents that have to be introduced, via our food, in small quantities because they are not synthesized by the human body. The vitamin composition of infant formula is critical for correct infant development, particularly if the mother is unable to breast-feed and formula is the primary source of nutrition. Official analytical methods for the determination of water-soluble vitamins are based on procedures, mainly microbiological assays, which have been established for decades Each vitamin is analyzed separately in order to apply extraction conditions, which permit the determination of its total content in a food. Vitamin analysis in food is generally a time-consuming process.
In this application note, we describe a rapid, five-minute UPLC-MS/MS method using positive ESI ionization for the simultaneous analysis of 12 water-soluble vitamin compounds in infant formula.
This method allows for the simultaneous analysis of 12 water-soluble vitamin compounds:
Vitamins are minor constituents that have to be introduced, via our food, in small quantities because they are not synthesized by the human body. The vitamin composition of infant formula is critical for correct infant development, particularly if the mother is unable to breast-feed and formula is the primary source of nutrition. Official analytical methods for the determination of water-soluble vitamins are based on procedures, mainly microbiological assays, which have been established for decades1,2. Each vitamin is analyzed separately in order to apply extraction conditions, which permit the determination of its total content in a food. Vitamin analysis in food is generally a time-consuming process.
The development of a single method for their simultaneous determination of vitamins in fortified infant formula is difficult for several reasons:
In this application note, we describe a rapid, five-minute UPLC-MS/MS method using positive ESI ionization for the simultaneous analysis of 12 water-soluble vitamin compounds in infant formula.
Throughout the sample preparation and analyses, all solutions were protected from exposure to light and stored at <5 °C.
Standard solutions of the vitamin compounds were prepared fresh daily.
|
LC System: |
ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC HSS T3, 1.8 μm, 2.1 x 50 mm |
|
Column temp: |
40 °C |
|
Sample temp: |
4 °C |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
10 mM Ammonium formate in water + 0.1% formic acid |
|
Mobile phase B: |
10 mM Ammonium formate in methanol + 0.1% formic acid |
|
Total runtime: |
5.0 min |
|
Injection volume: |
10 μL, full loop |
|
Time (min) |
%A |
%B |
|---|---|---|
|
0.0 |
99.0 |
1.0 |
|
2.0 |
99.0 |
1.0 |
|
3.0 |
45.0 |
55.0 |
|
3.1 |
1.0 |
99.0 |
|
4.0 |
99.0 |
1.0 |
|
5.0 |
99.0 |
1.0 |
|
MS System: |
Xevo TQ MS |
|
Ionization: |
ESI positive |
|
Capillary voltage: |
1.0 kV |
|
Source temp: |
150 °C |
|
Desolvation temp: |
600 °C |
|
Desolvation gas: |
1200 L/hr |
|
Acquisition: |
Multiple Reaction Monitoring (MRM) with RADAR full scan Collision gas: Argon at 3.5 x 10-3 mbar |
Since blank samples were not available, quantitative analysis of water soluble vitamins in infant formula powders was performed by the standard-addition method (except for nicotinamide)*. An analyte solution of known concentration (standard solution) was added to the sample so any matrix effects were accounted for in the calibration. The analyst did not know the amount of analyte in the sample initially, but tracked how much standard solution was added, and how the instrument response changed after adding the standard solution. Thus, by extrapolation of the calibration curve, the concentration of analyte in the sample could be determined. In practice, the volume of standard solution added is kept small to avoid dilution of the sample matrix.
* Using the current dilution factor (see experimental steps below) for the sample matrix without addition of standards, the instrument response of nicotinamide (B3) initially present in the infant formula is close to reaching the saturation of the detector. If standard-addition method is used for nicotinamide, detector saturation will be reached.
Prepare the infant formula to three times the concentration described on the packaging.
Place 10 mL of infant formula solution into a PP tube covered with foil; add 20 mL of 100% ethanol.
Shake vigorously for 2 min, and centrifuge for 15 min at 3500 RPM.
Filter supernatant using a 0.45 PVDF filter.
Transfer 20 µL of the supernatant into an amber autosampler vial, add 10 µL of known concentrations of standards containing 11 analytes, and top off the autosampler vial to 1 mL with water (the analyte in sample matrix was diluted 50 times; analyte in standard was diluted 100 times in final volume).
A separate standard curve of nicotinamide in solution was prepared.
Analyze by LC-MS/MS.
Data were acquired using MassLynx Software, v.4.1, and processed using TargetLynx Application Manager.
IntelliStart Technology was used to automatically develop fully optimized MRM acquisition methods for the 12 vitamin compounds targeted in this analysis. IntelliStart requires only the entry of basic compound information, and automatically locates the precursor ion, optimizes cone voltage, locates product ions, and optimizes collision energy.
Two MRM transitions were optimized for each vitamin compound; the first transition for quantitation and the second transition for confirmation. The dwell times for the transitions were automatically optimized to give a minimum of 12 points across each chromatographic peak for reproducible quantitation. The MRM transitions, cone voltages, and collision energies for the analyzed compounds, along with expected retention times, are shown in Table 1.
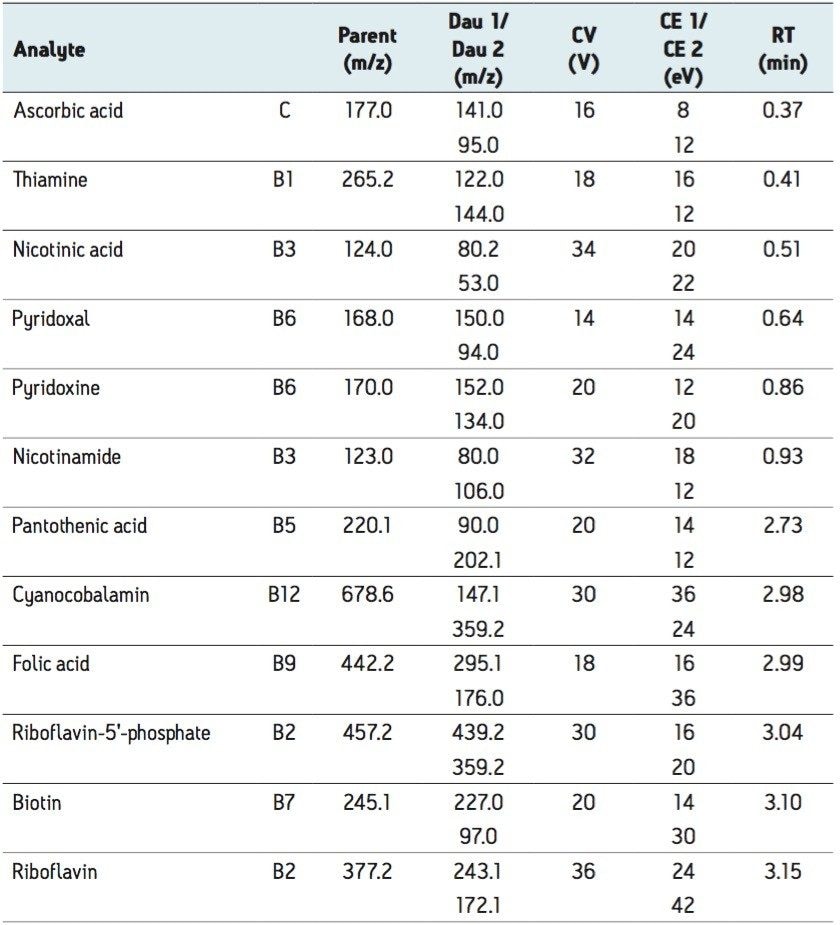
In addition to MRM data, full scan data were acquired using the RADAR mode of the Xevo TQ MS. RADAR is an information-rich acquisition approach that enables real time acquisition of spectral information on background components in the sample matrix, while simultaneously collecting MRM data. The use of RADAR does not compromise the quality of the MRM data.
12 water-soluble vitamin compounds were successfully analyzed using the ACQUITY UPLC System, coupled with Xevo TQ MS under ESI positive ionization. The use of ACQUITY UPLC enabled rapid separation of all analytes in <5 min, including 1 min for equilibration, as shown in Figure 1.
In the same analysis, full scan spectra to assess the background components in the infant formula matrix were also monitored using the RADAR mode of the Xevo TQ MS. RADAR utilizes the fast acquisition rates of the Xevo TQ MS, allowing full scan MS data to be acquired, while still collecting a sufficient number of points across the analyte peak, in MRM mode, for accurate quantification and confirmation.
With RADAR, the analyst can observe untargeted contaminants in the sample matrix, and get an idea of the level and type of compounds causing possible matrix effects. This provides insight in the development of matrix reduction strategies during LC-MS/MS methods development. For example, the separation method could be modified to move the peaks of interest away from where areas of potential matrix effects or ion suppression are likely to be present. An illustration of a MS spectrum extracted from a section of the full scan data of the infant formula sample matrix is shown in the insert of Figure 1.
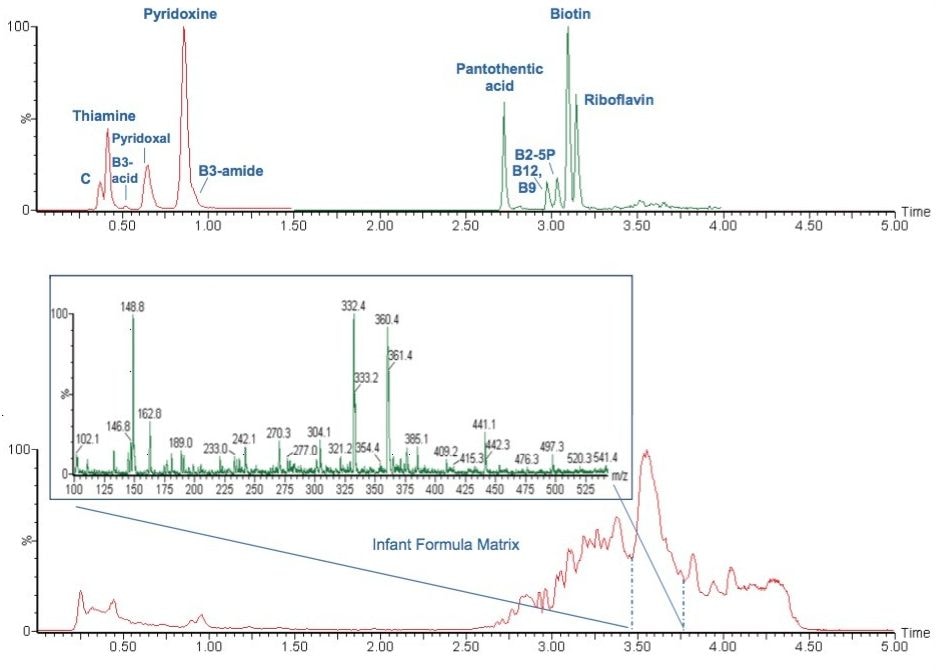
This method was tested on two different brands of publically available infant formula powder. Figure 2 shows the extracted quantifier ion chromatograms for the water-soluble vitamins detected in one brand of infant formula powder.
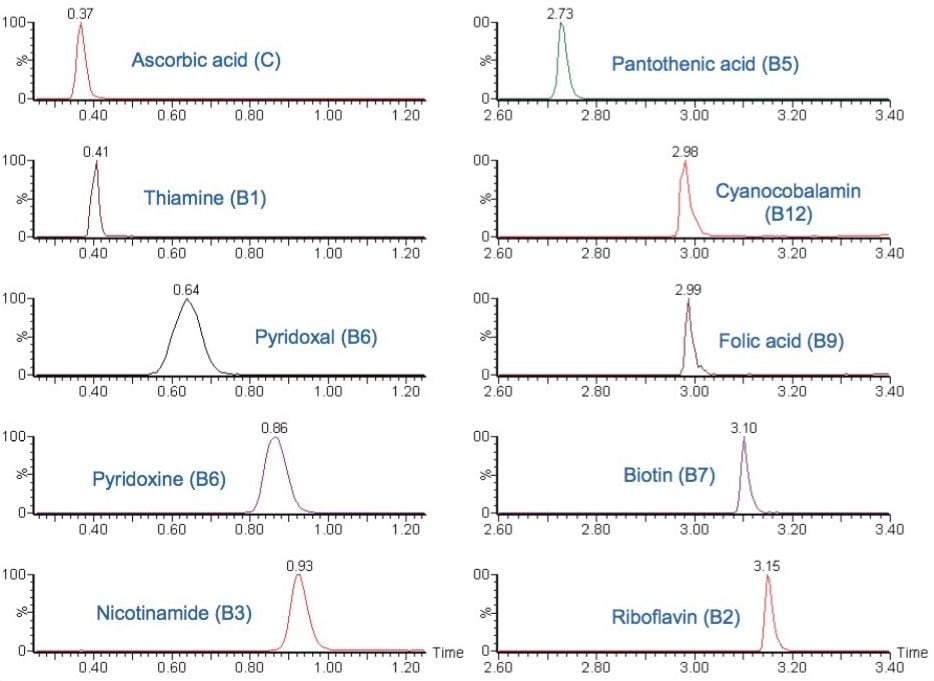
Recovery was determined by comparing the pre-extracted spiked samples with the post-extracted spiked samples. Experiments were performed for two different brands of infant formula powders on two different days, with six replicates being performed on each day.
For both brands of infant formulas, extraction recoveries of ≥ 90% were generally achieved for most of the compounds, with the exception of pyridoxal, folic acid, and riboflavin-5’-phosphate, whose lower recoveries could most probably be due to their poor solubility.
Despite infant formula powder being a very complex matrix, RSDs of less than 5% were attained for six replicates. This shows high reproducibility and robustness of the proposed solution.
Linear dynamic range, sensitivity, and suitability of the linear model were evaluated by applying the standard-addition method. An advantage of the standard-addition method is the avoidance of the evaluation of the matrix effect, responsible for signal ion suppression.
Excellent linearities were observed with correlation coefficients ≥ 0.99, as shown in Table 3, for all of the analytes in infant formula tested, over wide concentration ranges: 10 to 10000 ng/mL for ascorbic acid (C); 0.1 to 10.0 ng/mL for cyanocobalamin (B12); and 1 to 100 ng/mL for the rest of the analytes. Calibration curves of ascorbic acid (C), and cyanocobalamin (B12), based on standard-addition in one brand of infant formula powder are shown in Figure 3.
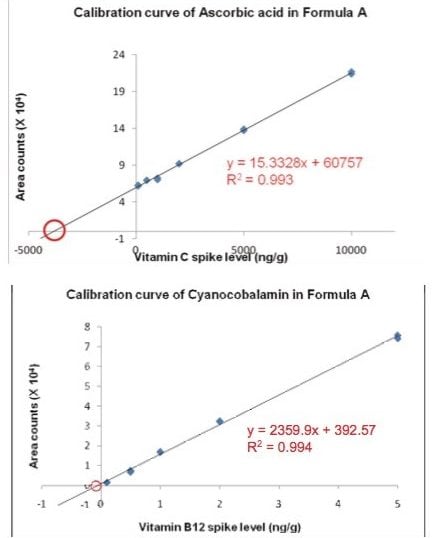
The calibration curve was extrapolated, and the absolute value of the x-intercept showed the concentration of the analyte in the infant formula, after taking into account the dilution factor (50 x) that was used. The sensitivity of the Xevo TQ MS allowed us to dilute the sample to reduce matrix effects, while still detecting the target compounds with confidence.
A rapid 5-minute method using ACQUITY UPLC with Xevo TQ MS in positive ESI ionization mode was developed for the simultaneous analysis of 12 water-soluble vitamin compounds. This method replaces individual, lengthy methods for vitamin analysis. By combining separate vitamin analyses into a single run, laboratories can increase sample analysis throughput, reduce solvent consumption, and decrease their operational costs.
With the sensitivity of the Xevo TQ MS, it is possible to detect target compounds at low concentrations (particularly cyanocobalamin), in a very complex matrix, such as infant formula powder. With the low limit of quantification achievable on the Xevo TQ MS, samples can be diluted to reduce matrix effects.
RADAR Technology allows for monitoring of matrix interferences, impurities, and degradants in samples, while accurately quantifying target compounds. This allows analysts to make informed decisions when assessing matrix effects, and enables a true assessment of whether matrix effects are likely to be present.
IntelliStart Technology simplifies system setup and MRM methods development, ensuring scientists of all levels can operate the instrument quickly and confidently, and start generating reproducible UPLC-MS/MS data of the highest quality.
720003694, September 2010