
There are many parameters available to the chromatographer in developing methods for separation of proteins. The approach to making adjustments to a method must take into consideration the objective of the analysis.
Due to the resolving power that reversed-phase chromatography provides, it has long been a preferred analytical technique to characterize and quantify various products. With an ever increasing emphasis on protein biopharmaceuticals, there is a need to develop reversed-phase separations of these macromolecules. Reversed-phase separations for proteins are not as powerful as they are for small molecules. Changes to the protein are often small in proportion to the structure of the large molecule. Variant forms, therefore, have similar chromatographic properties. There are still many factors that can be used to optimize the separation of a particular sample. The requirements of this specific application will dictate the best approach for method development. This paper will consider each of these factors, including particle size, column length, flow rate, modifier concentration, organic solvent, column temperature, and gradient slope. The evaluation of each of these method variables will be demonstrated on a variety of proteins, including monoclonal antibodies, covering a wide range of properties. These include differ-ent isoelectric points, hydrophobicities, and molecular weights.
Prepared in 5% acetonitrile with 0.1% CF3COOH
Intact Murine IgG1, prepared in 0.1% CF3COOH, 0.5 μg/μL. Reduced/Alkylated Murine IgG1, prepared in 0.1% CF3COOH, 0.5 μg/μL. Intact IgG Mixture: Humanized IgG4, Chimeric IgG1, and Murine IgG1, prepared in 0.1% CF3COOH, 0.5 μg/μL each.
Vials:
Waters Certified Total Recovery
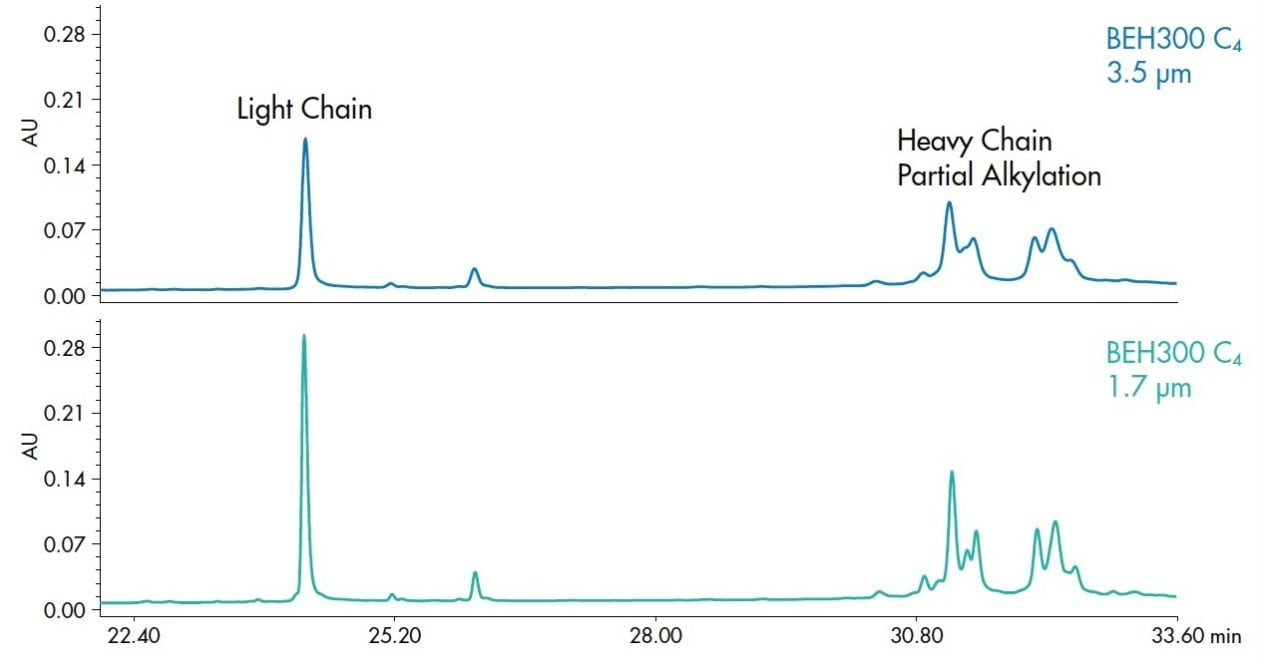
The advent of sub-2 µm particles along with UPLC Technology has shown benefits for samples of all types. This technology was applied for the separation of biological macromolecules with the development of BEH300 C4, a column chemistry that com-bines appropriate pore volume and chain length on a hybrid particle.1 This stationary phase is available in 3.5 μm and 1.7 μm particle sizes, so methods can be directly transferred between HPLC and UPLC with the same chromatographic selectivity. Figure 1 shows the comparison of the effect of particle size, using a reduced and partially alkylated IgG sample. The relative positions of the peaks are exactly the same for both particle sizes. Since both separations were tested on the same UPLC system with the same mobile phases and conditions, the improvement in resolution observed for the 1.7 µm separation is directly attributable to the smaller particle size. The rigorous manufacturing control in the particle synthesis ensures scalability and constant selectivity across particle sizes. The improvement in resolution can only be fully realized with use of a system designed to minimize band-broadening during the separation.
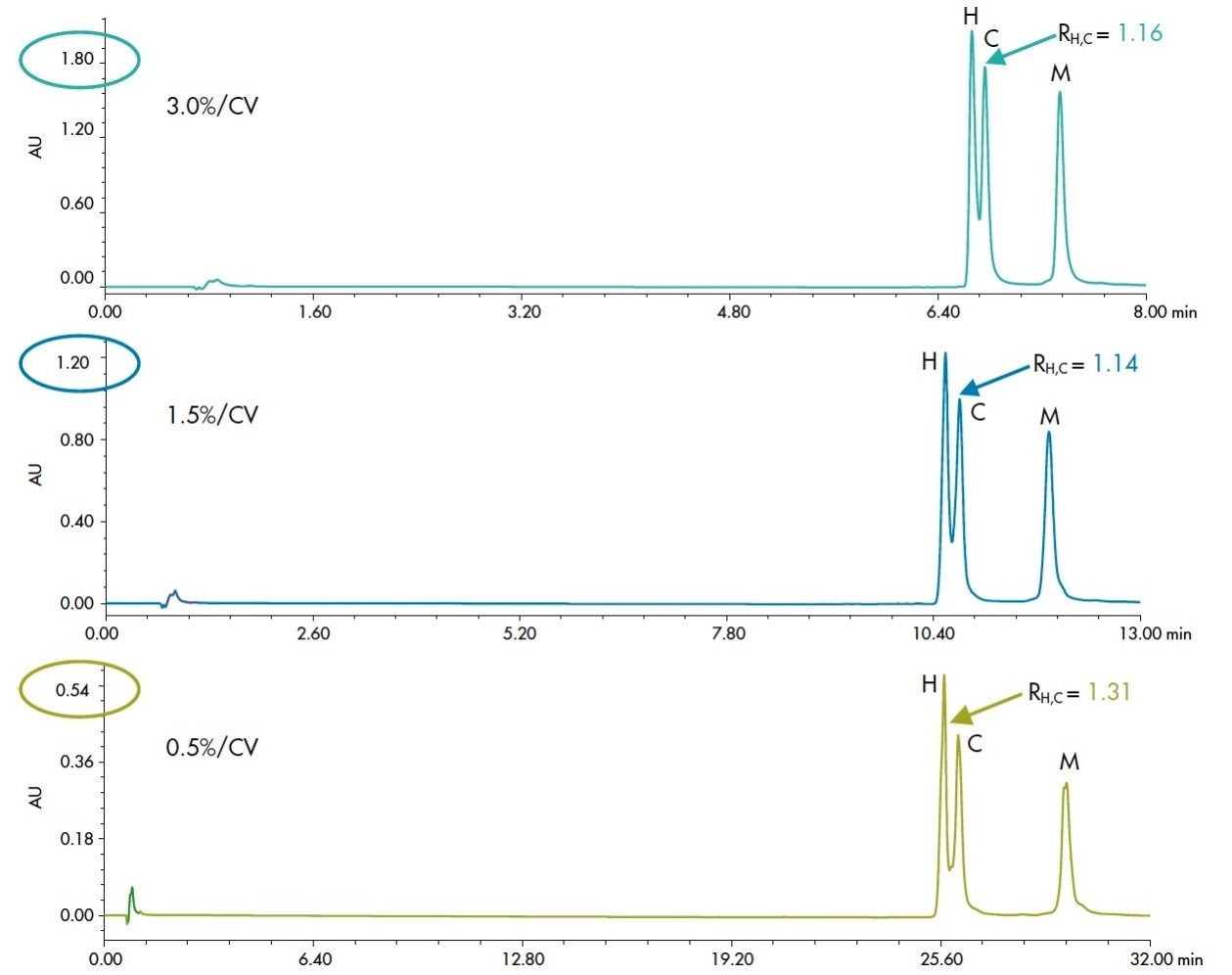
In gradient separations, chromatographers will often change the gradient slope as a primary tool in method development. Gradient slope, defined by the percent increase in organic per column volume, can be adjusted to optimize a separation for resolution of components or speed of analysis. Typical protein separations use fairly shallow gradients of about 3% or less. Reducing the gradient slope does offer an increase in resolution. Sensitivity, however, is reduced as the gradient is made more shallow. The resolution improvement in protein separations is usually at a slower rate than the loss of sensitivity or increase in peak volume. This phenomenon can be seen readily in the separation of a mixture of IgG, shown in Figure 2. By reducing the gradient slope from 3% to 0.5%, there is only a marginal increase in resolution between the humanized and chimeric IgG peaks, while there is over a 3-fold loss of sensitivity and a 4-fold increase in run time. While gradient slope is a viable tool in method development, it is preferred to reserve that option until other techniques have been examined.
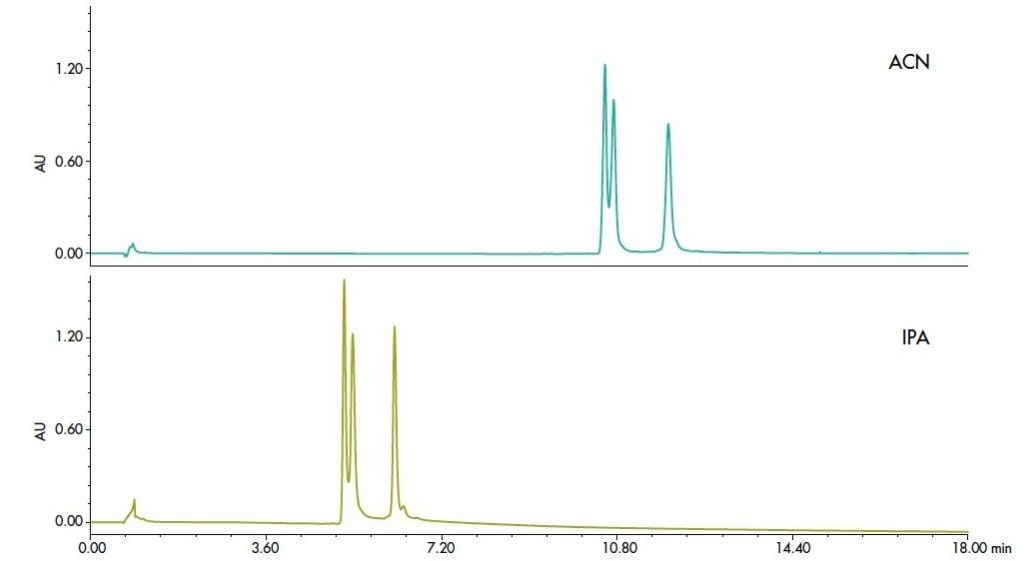
Alternative organic solvents can alter selectivity of a separation. Historically, acetonitrile has been the solvent of choice in protein separations. The use of isopropanol (IPA) has been common. Gradients of increasing IPA were seldom used because of the high pressure associated with the viscosity of such solvent mixtures. Therefore, an acetonitrile/isopropanol blend (3:7) was a preferred replacement. The higher pressure capability of ACQUITY UPLC instruments allow for use of 100% isopropanol, as shown in Figure 3. All of the proteins elute earlier with IPA, and for this sample, improved resolution of some minor components is observed.
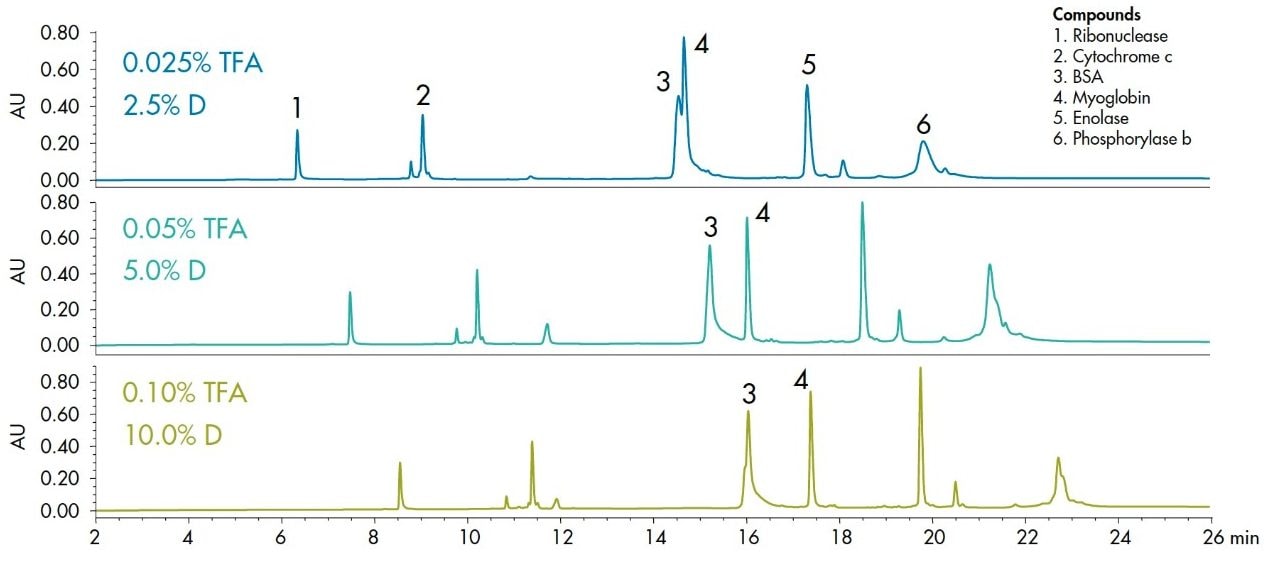
Type and concentration of the acid modifier can also influence the separation. Formic acid is the preferred modifier in mass spectrometry applications, and trifluoroacetic acid (TFA) gives better chromatographic peak shape. Altering the acid concentration can change the selectivity of the separation. In general, protein peaks elute earlier with lower trifluroacetic acid (TFA) concentration, reflecting the reduced ion pairing.
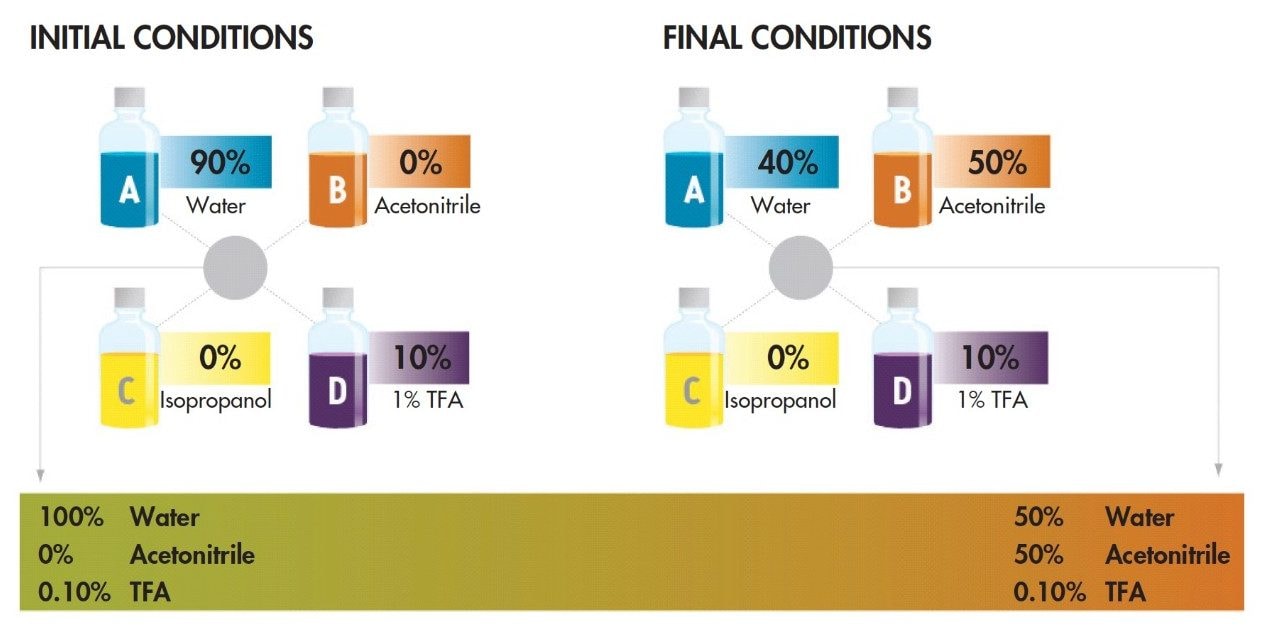
There are a large number of organic solvent and acid concentration combinations possible in the develop-ment of a separation. This process can be streamlined with the application of Auto•Blend Technology, as embodied on the four solvent ACQUITY UPLC H-Class system. Figure 4 shows the preferred configuration of the system for testing the effect of organic solvent and mobile-phase modifier concentration on a protein separation. The conditions to be tested are pro-grammed in the method as percentage flow from each of the four solvent lines. For example, different TFA concentrations are tested by blending a concentrated acid modifier at a series of percentages. This approach was used for the protein separation shown in Figure 5. While all of the peaks elute earlier at lower concentra-tions of TFA, myoglobin elutes earlier relative to the other proteins. It should also be noted that lower TFA concentration also results in generally wider peaks, which can lead to lower resolution, as can be seen with the different forms of phosphorylase b. Altering modifier concentration can be a very useful tool in method development, particularly where changes in selectivity are needed. The same approach can be used to compare different organic solvents.
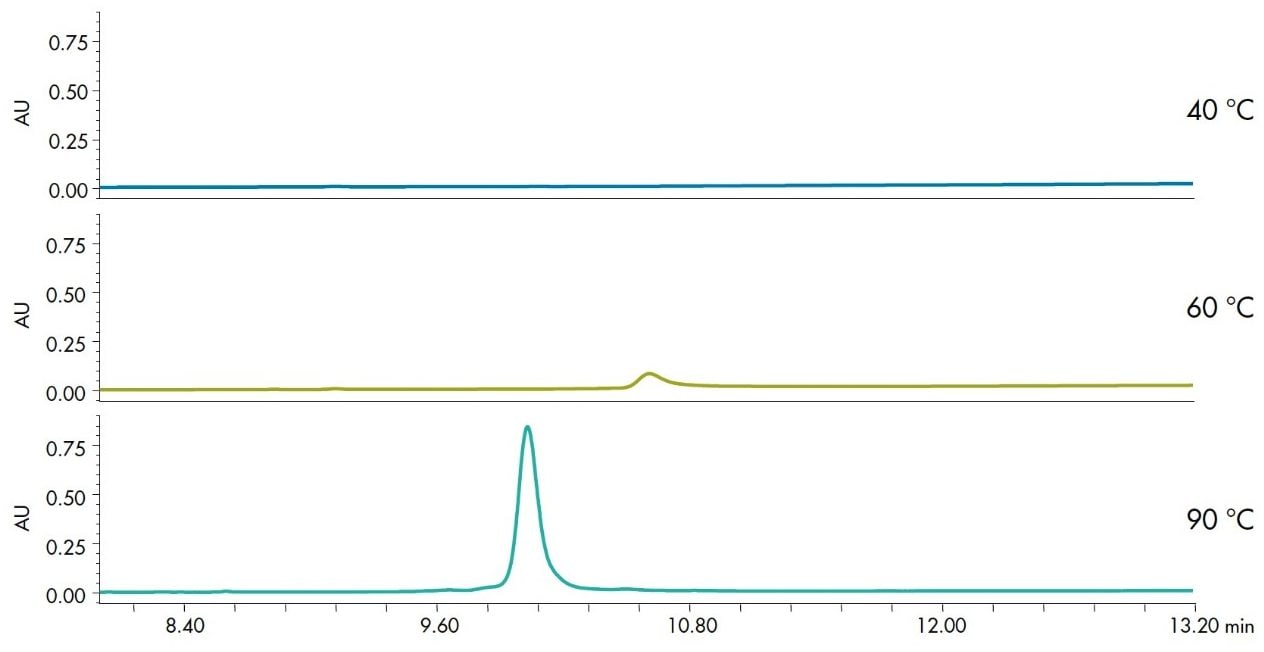
Column temperature has a large effect on reversed-phase separation of molecules. Changes in recovery and selectivity are not uncommon with small molecule separations. While increasing the temperature for proteins can significantly improve recovery, particularly for intact monoclonal antibodies (Figure 6), it doesn’t generally affect the selectivity of the separation.2 However, not all proteins require higher temperatures for improved recovery. In fact, some protein separations have more desirable results with lower separation temperatures. Therefore, it is recommended that an evaluation of temperature be included in any method development strategy for new samples.
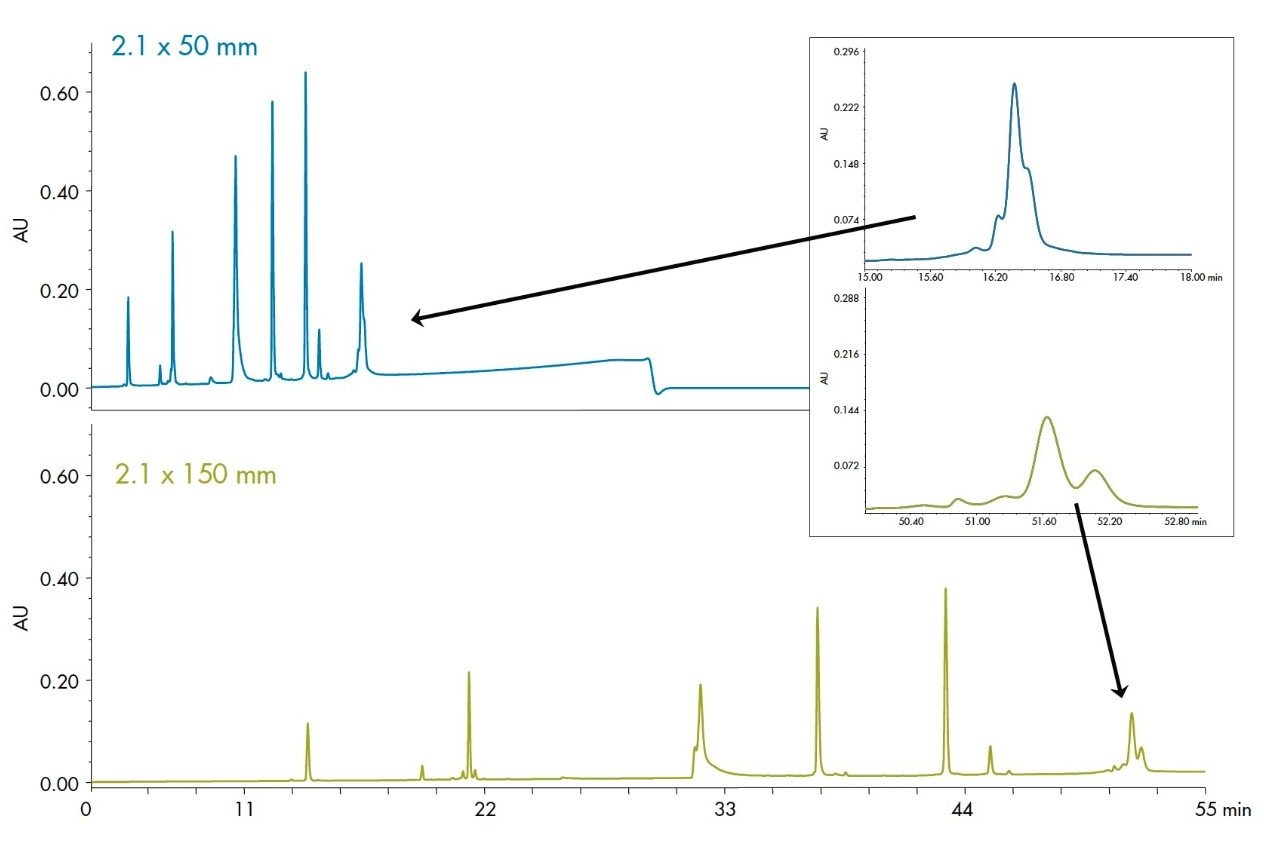
Increasing the length of the column will increase the resolving power for a separation. This is shown in Figure 7 with the separation of the protein mixture. The additional small peaks surrounding the Phosphorylase b can be seen more readily on the longer column, as seen in the inset, but it comes at the cost of a 3-fold increase in run time and ~40% loss of sensitivity. Depending on the application objective, this may be a useful parameter to improve resolution.
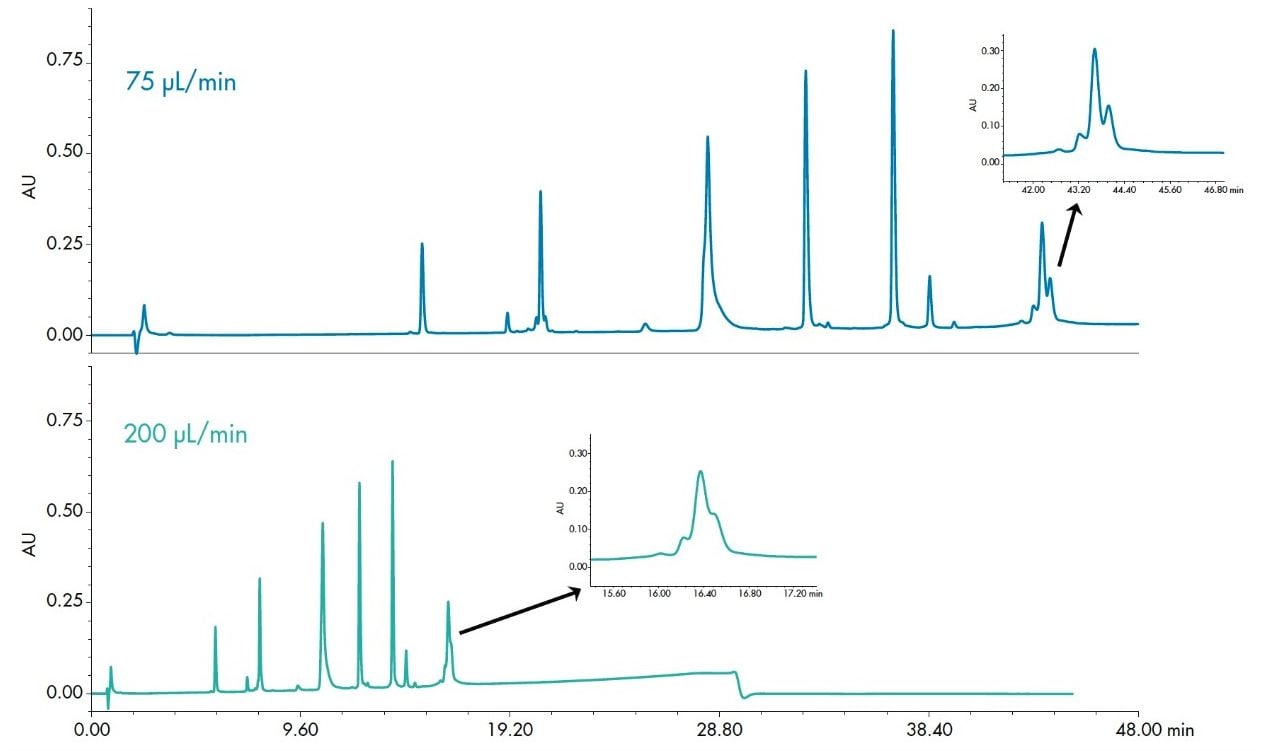
Flow rate is seldom treated as an important parameter in method development except as an indirect modification of gradient slope. The impact of this variable is, however, more significant for larger molecules. Figure 8 shows the comparison of a protein mix separation at 200 μL/min and 75 μL/min. The inset shows improved resolution with an increase in sensitivity of phosphorylase b at the lower flow rate.
There are many parameters available to the chromatographer in developing methods for separation of proteins. The approach to making adjustments to a method must take into consideration the objective of the analysis.
Protein separations do not tend to show the same dramatic resolution effects seen for small molecules. Therefore, most of the variables discussed here yield small improvements, often at the expense of sensitivity and run time. Smaller particle columns do, however, offer resolution improvements without loss of sensitivity or increased run time. Flow rate, column length, gradient slope, and modifier concentration can then be manipulated to further improve resolution.
Modifier concentration can be a useful tool in developing methods. It can provide resolution improvements by possible selectivity changes. Furthermore, changing the concentration can affect peak shape and detection.
Auto•Blend Technology is a convenient and efficient way to optimize systematically the effects of modifier concentra-tion and organic solvent selection on the separation.
Adjustment in column temperature does not usually provide much selectivity change, but it can have a significant impact on the peak shape and recovery of proteins. It is not always possible to predict the ideal temperature for a protein sample. Therefore, it is good practice to include multiple temperatures in evaluation of appropriate conditions for a protein separation.
Both increased column length and decreased flow rate give increased resolution, both at increased run time. However, decreasing flow rate does not compromise sensitivity, as is the case with the longer column. The longer column, however, permits the injection of a larger sample, which may be valuable in the analysis of trace components.
Benefits in sensitivity, resolution, and run time can be achieved with smaller particles. But these improvements are best realized when applied with the holistic design of the UPLC system.
720003875, May 2011