In this application note, we discuss the concepts to be considered to achieve high peak capacity nanoscale trap-elute peptide separations.
Nanoscale LC enables the analysis of minimal sample quantities with comparatively higher mass spectrometric sensitivity versus higher flow rate chromatographic separations, such as those achieved with narrow bore (2.1 mm I.D.) columns.1-2 Not surprisingly, studies aiming to characterize the proteolytic digests of complex biological systems have been made possible by modern nanoscale LC techniques. Notable examples include the use of nanoLC-MS to elucidate cellular mechanisms and to discover biomarkers.3-4
With nanoLC, however, it is generally not reasonable to analyze large volume samples using just a single column. Instead, it is preferable to perform online trapping of analytes at microscale flow rates and to subsequently elute and separate those analytes across an analytical column, wherein a significantly lower nanoscale flow rate is employed. Often, tens of microliters of sample must be analyzed, and in these samples, analytes may be present at concentrations differing by more than several orders of magnitude. Performing trapping on a trap column amenable to high flow rates allows for greater throughput efficiency as well as desalting in a step that diverts unwanted solutes away from a downstream mass spectrometer without adding additional connections and tubing after the analytical column (Figure 1). A trap column can also function as a guard column to preserve the performance of the analytical column. Beyond its role as a guard column, an effective trapping column is packed with larger diameter particles, typically 5 µm, to enable higher velocity loading and retention of large, possibly dilute sample volumes. To be effective, however, a trap column should have lower retentivity when compared to the analytical column. This match between trapping and analytical columns during gradient elution ensures refocusing of analytes on to the analytical column and that high peak capacity separations are delivered to the MS detector.
In this application note, we discuss the concepts to be considered to achieve high peak capacity nanoscale trap-elute peptide separations. A 15K psi capable, ACQUITY UPLC M-Class System is used to demonstrate high peak capacity trap-elute peptide separations with a charge surface hybrid (CSH) C18 analytical column.
(unless otherwise noted):
For the study of peptide retention:
|
System: |
ACQUITY UPLC H-Class System Bio with a 20-cm Column Heater |
|
Detection: |
ACQUITY UPLC TUV |
|
Detector Wavelength: |
214 nm |
|
Scan rate: |
10 Hz |
|
Columns: |
XSelect Peptide CSH C18, 130Å, 3.5 μm, 2.1 x 50 mm (p/n 186006950) XBridge Peptide BEH C18, 130Å, 3.5 μm, 2.1 x 50 mm (p/n 186003563) Symmetry C18 Column, 100Å, 3.5 μm, 2.1 x 50 mm (p/n WAT200650) XSelect HSS T3 Column, 100Å, 3.5 μm, 2.1 x 50 mm (p/n 186006463) |
|
Column temp.: |
30–80 °C |
|
Sample temp.: |
5 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.2 mL/min |
|
Mobile phase A: |
0.1% formic acid (v/v), water |
|
Mobile phase B: |
0.1% formic acid (v/v), acetonitrile (ACN) |
|
Vials: |
LCGC Certified Clear Glass 12 x 32 mm Screw Neck Qsert Vial (p/n 186001126C) |
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
|---|---|---|---|
|
0 |
100 |
0 |
0.2 |
|
30 |
60 |
40 |
0.2 |
|
32 |
10 |
90 |
0.2 |
|
35 |
10 |
90 |
0.2 |
|
36 |
100 |
0 |
0.2 |
|
45 |
100 |
0 |
0.2 |
|
MS system: |
Xevo G2 QToF |
|
Ionization mode: |
ESI+ |
|
Analyzer mode: |
Resolution |
|
Capillary voltage: |
3.0 kV |
|
Cone voltage: |
25 V |
|
Source temp.: |
100 °C |
|
Desolvation temp.: |
350 °C |
|
Cone gas flow: |
0.0 L/Hr |
|
Desolvation gas flow: |
800 L/Hr |
|
Calibration: |
NaI, 2 μg/μL from 50–2000 m/z |
|
Acquisition: |
50–1990 m/z, 10 Hz scan rate |
|
Data management: |
MassLynx Software (v4.1) |
|
For the development of nanoscale trap-elute |
|
System: |
ACQUITY UPLC M-Class System with a Trap Valve Manager (and heater) and a CH-A 20-cm Column Heater (p/n 186015042) |
|
Analytical Column: |
ACQUITY UPLC M-Class CSH C18, 130Å, 1.7 μm, 75 μm x 150 mm (p/n 186007476) |
|
Trapping column: |
ACQUITY UPLC M-Class Symmetry C18, V/V, 100Å, 5 μm, 180 μm x 20 mm (p/n 186007500) |
|
Analytical column temp.: |
30-60 °C |
|
Trapping column temp.: |
Ambient - 60 °C |
|
Sample temp.: |
5 °C |
|
Injection volume: |
0.2 μL (intermediate mass load), 2 μL (high mass load) |
|
Flowrate: |
15 μL/min (trapping), 0.5 μL/min (analytical) |
|
Mobile phase A |
0.1% formic acid (v/v), water |
|
Mobile phase B: |
0.1% formic acid (v/v), acetonitrile (ACN) |
|
Weak needle wash: |
0.1% trifluoroacetic acid (TFA) (v/v), water |
|
Vials: |
LCGC Certified Clear Glass 12 x 32 mm Screw Neck Qsert Vial (p/n 186001126C) |
|
Trapping: |
Trapping column effluent diverted to waste |
|
Analytical gradient: |
Trapping column in-line with analytical column |
|
Time (min) |
Flow Rate (μL/min) |
%A |
%B |
|---|---|---|---|
|
0 |
99.5 |
0.5 |
15 |
|
1 |
99.5 |
0.5 |
15 |
|
Time (min) |
Flow Rate (μL/min) |
%A |
%B |
|---|---|---|---|
|
0 |
99.5 |
0.5 |
0.5 |
|
60 |
60.0 |
40.0 |
0.5 |
|
62 |
15.0 |
85.0 |
0.5 |
|
65 |
15.0 |
85.0 |
0.5 |
|
68 |
99.5 |
0.5 |
0.5 |
|
90 |
99.5 |
0.5 |
0.5 |
|
MS system: |
SYNAPT G2-S (with NanoLockSpray ion source) |
|
Ionization mode: |
ESI+ |
|
Analyzer mode: |
Resolution |
|
Capillary voltage: |
3.0 kV |
|
Cone voltage: |
30 V |
|
Source offset: |
50 V |
|
Source temp.: |
100 °C |
|
Nanoflow gas pressure: |
1.5 Bar |
|
Calibration: |
NaI, 2 μg/μL from 100–2000 m/z |
|
Acquisition: |
50–1900 m/z, 2 Hz scan rate |
|
Data management: |
MassLynx Software (v4.1) |
For the study of peptide retention on C18 stationary phases, MassPREP Peptide Mixture (p/n 186002337) was mixed with Peptide Retention Standard (p/n 186006555) to make a solution in 0.1% formic acid, water that was comprised of ~20 pmol/µL MassPREP peptide mixture and 170 pmol/µL Peptide Retention Standard.
For the development of nanoscale trap-elute separations, MassPREP Enolase Digest with Phosphopeptides Mix (p/n 186003286) was reconstituted in 200 µL of 0.1% (v/v) TFA in water to make a solution of 5 pmol/µL.
When used for low pH, MS-friendly reversed-phase peptide separations CSH C18 can deliver ~2-fold greater peak capacities when compared to conventional C18.5-8 It has additionally been shown that the performance of CSH C18 is largely related to its improved loadability.5,7 Interestingly, the performance advantages of CSH C18 can be attributed to its novel composition. CSH C18 is bonded with both a trifunctional C18 ligand and a controlled, low level coverage of basic residues. CSH C18 consequently exhibits a positive surface potential at acidic pH. This surface charge, estimated to be about +20 mV,9 repels similarly charged analytes, such as peptides at low pH, while minimizing undesired secondary interactions. But while the coloumbic repulsion between CSH C18 and peptides improves peak capacity and loadability, this improvement comes with a small compromise to retentivity. Compared to uncharged silica and hybrid-silica C18 columns, peptides tend to elute from CSH C18 columns in 2-4% less acetonitrile.5-8 Employing CSH C18 in a LC configuration involving just a single column simply requires adjustment of the initial gradient conditions to compensate for the reduced retentivity of the phase. In nanoLC, the use of a single column is referred to as direct injection. Wherever possible, direct injection should be favored in nanoLC as it is a simple configuration and the one most likely to produce the highest peak capacities. As mentioned before, it may nonetheless be preferable to perform nanoLC with a trap-elute configuration, such as one of those portrayed in Figure 1. In trap-elute separations, phase retentivity becomes very important. It is necessary that a trapping stationary phase exhibit a lower retentivity than the analytical column phase so that eluting peptides can be optimally refocused on the analytical column. In that way, a large particle (i.e. 5 µm) trapping phase can be used, keeping back pressures during trapping low, without detriment to the resolving power of the separation on the analytical column.
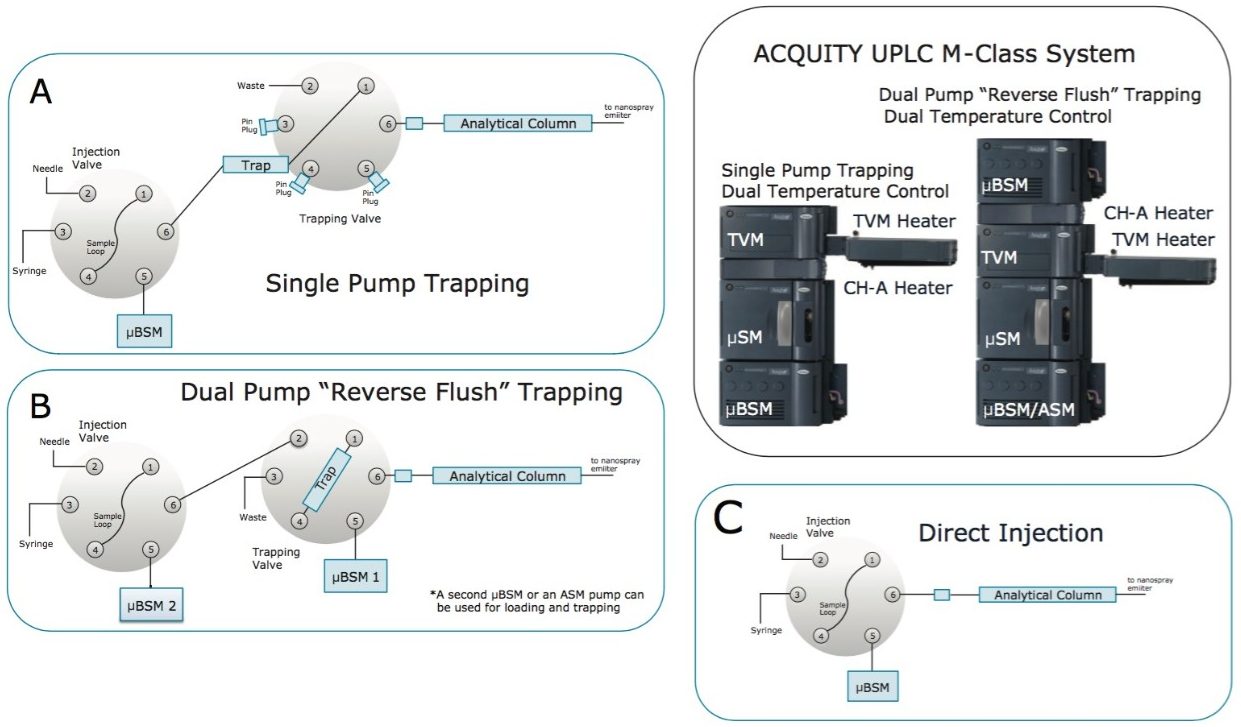
Recently, the combination of a 180 µm x 20 mm trap packed with 5 µm Symmetry C18 and a 75 µm I.D. analytical column packed with a C18 stationary phase, known as high strength silica (HSS) T3, was shown to be useful for nanoscale UPLC.10-14 Symmetry C18 is known to exhibit weak peptide retentivity, whereas HSS T3 exhibits a characteristically higher peptide retentivity. Trap-elute nanoLC where Symmetry C18 is combined with HSS T3 can thus be highly effective; that is, as long as low mass loads are injected. Looking to develop a trap-elute nanoLC separation capable of high mass loading, we consequently explored the considerations that must be made in order to optimize a trap-elute peptide separation in which the less retentive CSH C18 is used as the analytical phase instead of HSS T3.
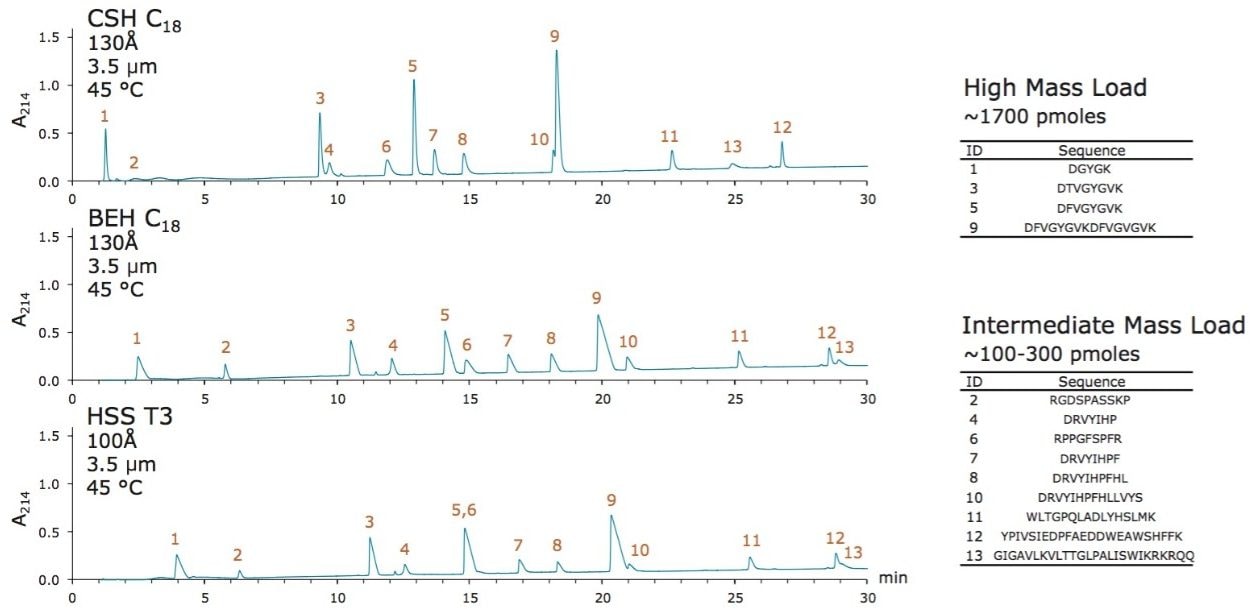
The ability of various stationary phases to retain peptides was investigated using narrow bore (2.1 mm I.D.) columns packed with 3.5 µm diameter particles, a test mixture composed of 13 different peptides, an ACQUITY UPLC H-Class Bio System, and a Xevo G2 QTof mass spectrometer. Figure 2 presents three chromatograms obtained with three common analytical column phases, CSH C18, BEH C18, and HSS T3. Many observations made previously during comparison of these phases are again observed in this work, namely differences in selectivity and loadability.5-8 The objective of this analysis was to catalog the retentivity of these phases. To this end, the retentivity of the mentioned analytical phases is reported in Figure 3A in terms of the acetonitrile composition needed to elute each individual peptide. To obtain additional information about the retentivity of these phases, their dependence on temperature was studied, from 30 °C up to 60 or 80 °C depending on the maximum suggested operating temperatures of the various phases. These same experiments were performed to study the retentivity of the Symmetry C18 phase, which is used in ACQUITY UPLC M-Class trapping columns (Figure 3A). Upon interpretation of these data, it becomes apparent that the retentivity of the phases can be ranked from low to high follows: Symmetry C18 < CSH C18 < BEH C18 < HSS T3. Moreover, it is clear that peptide retentivity decreases as column temperature increases. Figure 3B summarizes these observations as an average of the individual peptide retention measurements.
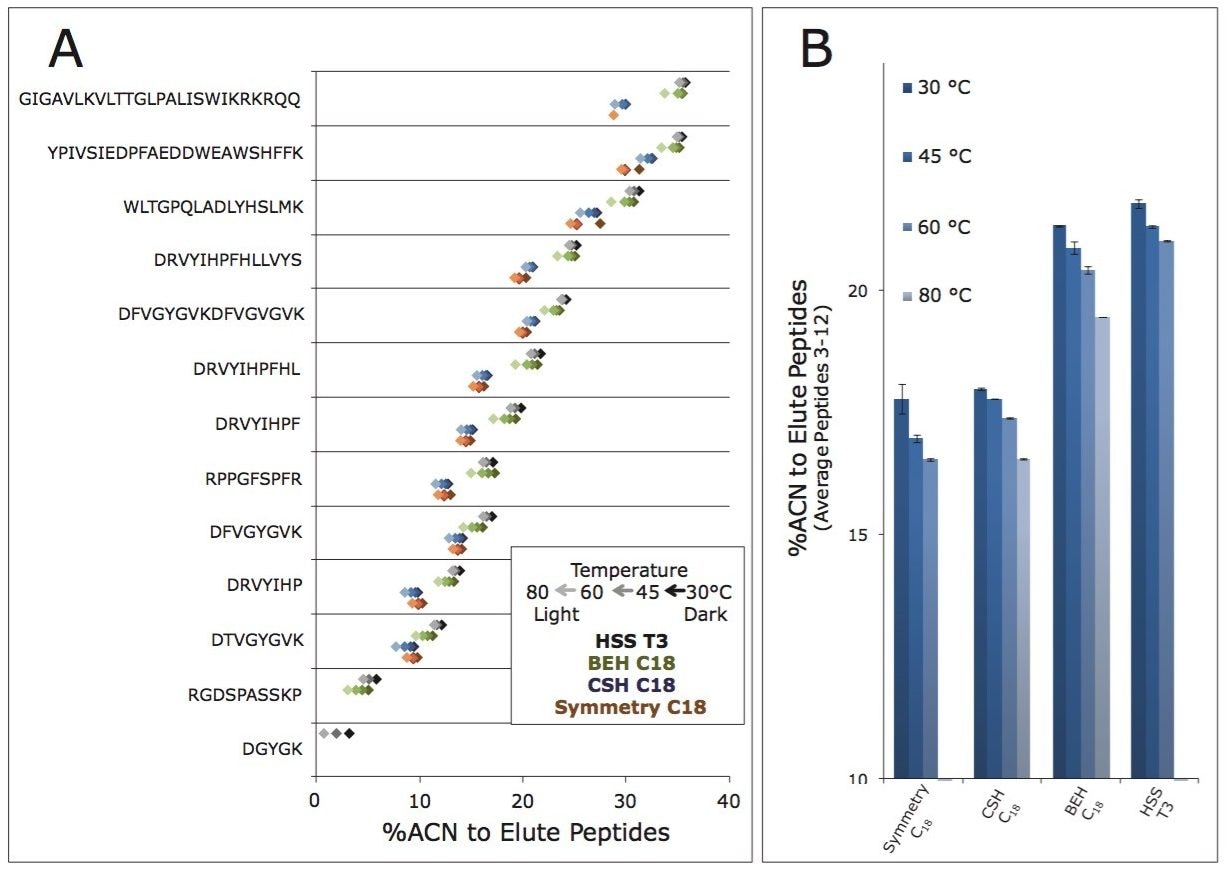
These results corroborate the effectiveness of pairing Symmetry C18 trapping with HSS T3 analytical separations. The two phases exhibit such different retentivities (∆% ACN >3%) that the analytical column can be operated over a wide range of temperatures independently of the trapping column. For instance, no matter the temperature at which the Symmetry C18 trap column is maintained, peptides will elute from it at a substantially lower elution strength than that required to elute the peptides from HSS T3. Peptides will consequently refocus on the head of the analytical column and elute as sharp detectable chromatographic peaks under a very broad range of experimental conditions. In contrast, the results shown in Figure 3 suggest that methods involving the pairing of Symmetry C18 trapping and CSH C18 analytical separations need to be more carefully controlled, due to the highly similar retentivity characteristics of the two phases, in order to ensure optimal analytical column focusing.
One way to optimize trap and analytical column retentivity is by independently controlling the temperature of the two devices. By adjusting phase retentivity through separately controlled column heaters, peptides can be induced to elute from a trapping column and re-focus on the analytical column. In standard configurations the ACQUITY UPLC M-Class System allows for a single heating zone. However, the system can add dual temperature control through addition of an ACQUITY Column Heater (CH-A, p/n 186015042), as shown in Figure 1. With such a setup, the trap column can be housed in the CH-A compartment at a temperature that is different from the analytical column residing in the standard ACQUITY UPLC M-Class heater.
Chromatographic separations of MassPREP enolase tryptic digest obtained with a Symmetry C18 trapping column and a CSH C18 analytical column in a typical trap-elute configuration (single-pump, forward elute, Figure 1A) are shown in Figure 4. In the example trap-elute separations, the analytical column was maintained at 30 °C, while the trapping column was heated at increasing temperatures of 30 °C, 45 °C, and 60 °C. Based on the peak widths of 5 enolase tryptic peptides, peak capacities were calculated for each scenario. A direct injection separation of this sample was found to yield a peak capacity (Pc,50% height) of 643. Meanwhile, these trap-elute separations yielded peak capacities of 322, 402, and 495, respectively. This observation indicates that phases with similar retentivity can indeed be optimized for use in both trapping and analytical columns. Maintaining the trapping column at a higher temperature than the analytical column reduces its retentivity, resulting in peptides eluting from the trap at a lower concentration of acetonitrile. Since the eluting peptides are delivered at lower percentage acetonitrile, the peptides are readily retained and focused on the analytical column. In turn, peak capacities more closely resembling direct injection can be achieved, while maintaining the advantages of trap-elute, such as being able to load and desalt large volumes of sample quickly.
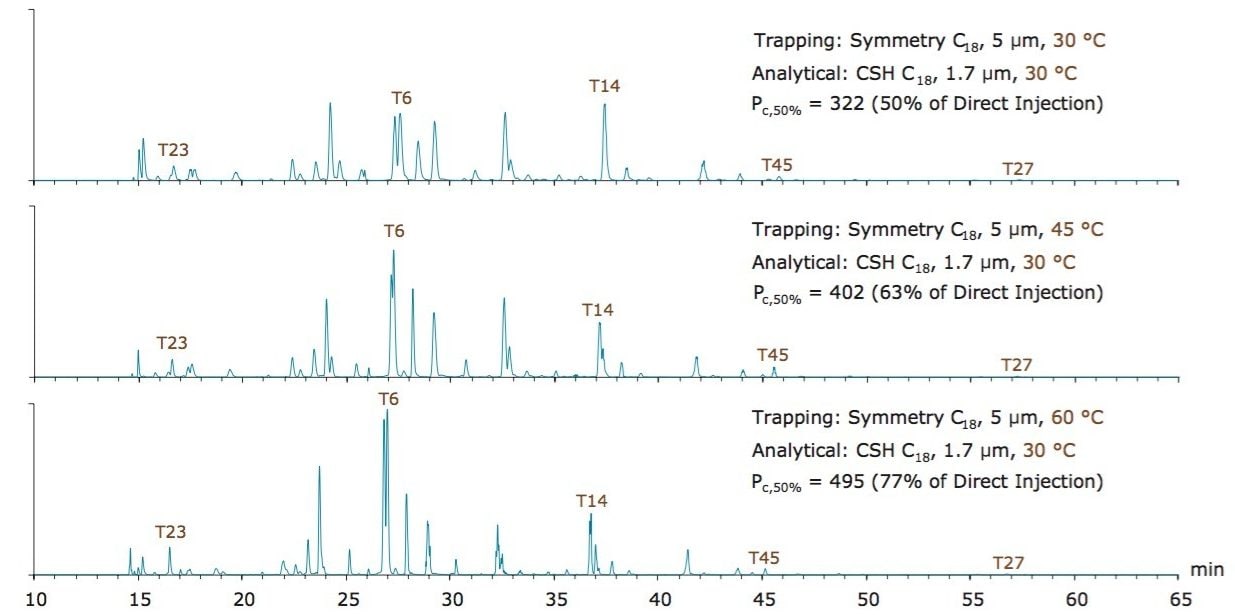
The ability to heat a Symmetry C18 trapping column up to 60 °C is clearly a useful asset to the development of high peak capacity peptide separations. Results displayed in Figure 4 demonstrate that the recoveries of weakly retained hydrophilic peptides are only marginally affected. Comparing the operation of the trapping column at 30 versus 60 °C, only 1 or 2 peptides were lost due to reduced trap retentivity. Moreover, stability testing of Symmetry C18 indicates that there is only a minor retentivity shift, corresponding to -0.05% ACN/day, due to incubation at 60 °C (Figure 5).
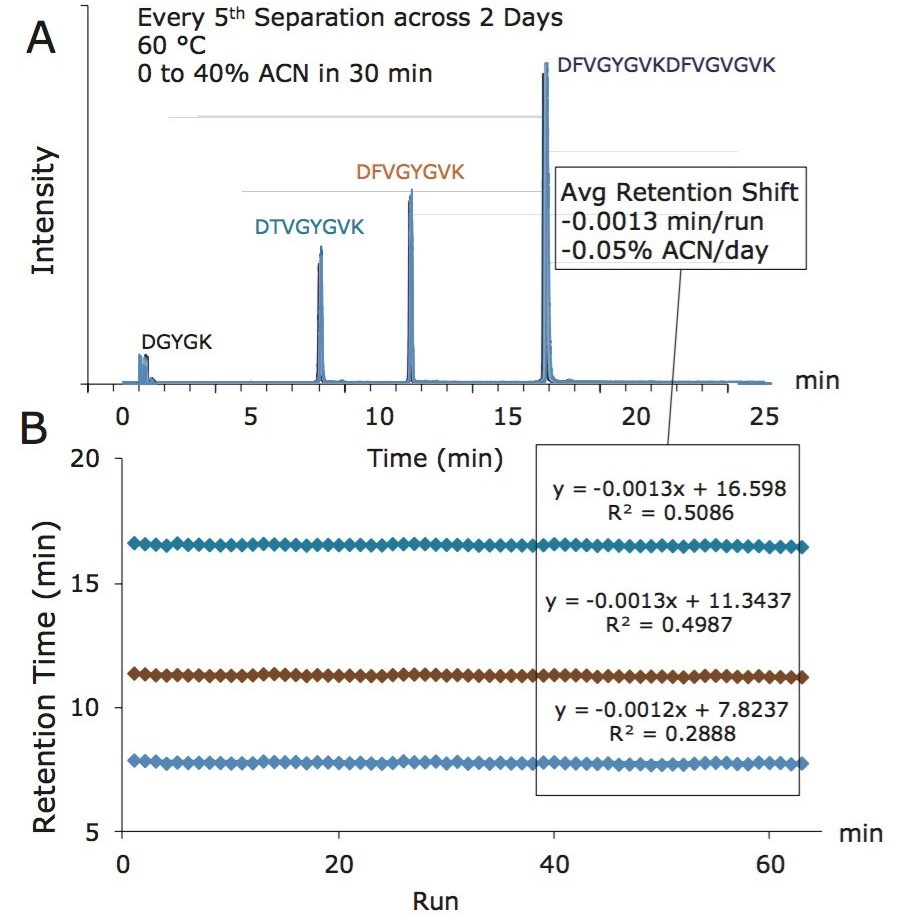
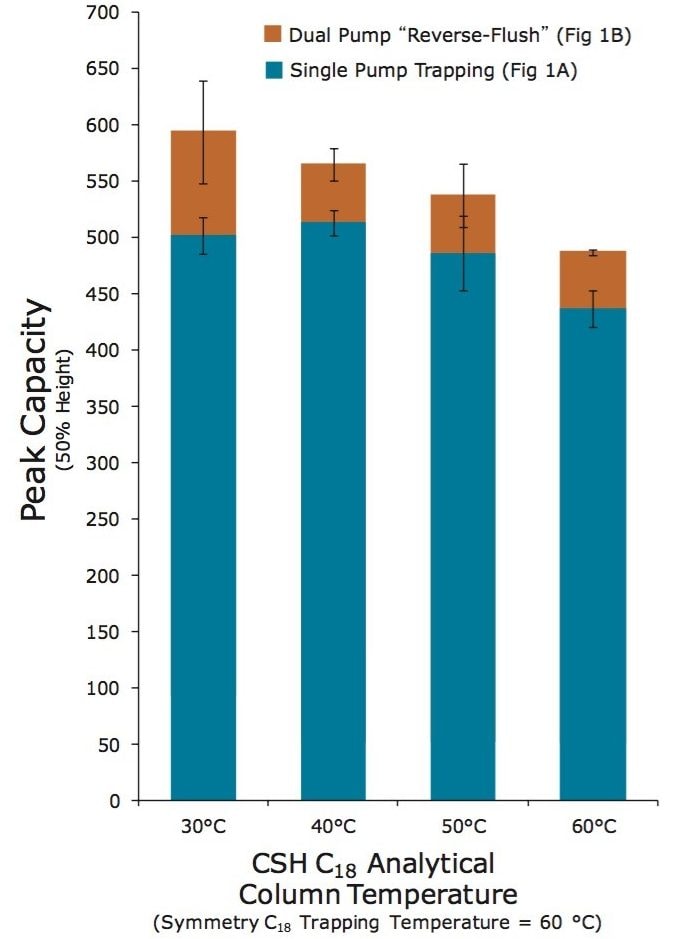
Having established the value and feasibility of operating a Symmetry C18 trapping column at 60 °C, it was important to optimize the temperature for best resolution of peptides from the CSH C18 analytical column. As before, peak capacities were measured based on the separation of an enolase digest using a single-pump, forward elute configuration. Unlike before, the trapping column was held at 60 °C, while the CSH C18 analytical column was subjected to increasing temperatures, from 30 to 60 °C. Peak capacities measured for these varying temperature settings are provided in Figure 6 (blue bars). The trend in the data indicates that a 60 °C Symmetry C18 trapping column pairs effectively with a CSH C18 analytical column operating between 30 and 40 °C. Despite this optimization though, the peak capacities obtainable with a single-pump, forward elute trap-elute configuration remain less than those possible by direct injection.
An alternative to the single-pump, forward elute configuration, was thus investigated, specifically the configuration shown in Figure 1B. While this configuration is more complicated, since it relies on a two pump system, it has notable advantages. In particular, this configuration makes it possible to load the trapping column in one direction and to elute its contents in the reverse direction. This so-called “reverse flush/elute” configuration can be exploited to obtain peak capacities more representative of the full capabilities of an analytical column as observed in direct inject mode. In this mode, analytes will not pass through the entirety of the trapping column and will not be subjected to the band broadening imparted by the 5 µm diameter particles and larger inner diameter hardware typical of a trapping column. Although outside of the scope of this work, it should also be noted that a two pump system such as this allows for the use of an ion pairing agent (e.g. TFA) to be used in the mobile phase of the trapping/loading pump. This has the effect to increase peptide retention/recovery without hindering ionization of peptides since they will be subsequently eluted in MS-friendly formic acid mobile phases delivered by the second pump system. In this work with only formic acid mobile phases, the reverse-elute configuration proved to yield significantly higher peak capacities (red bars, Figure 6). In fact, reverse-elute separations with a Symmetry C18 trap heated to 60 °C and a CSH C18 analytical column maintained between 30 and 40 °C were found to produce peak capacities within 10% difference of those obtained by direct injection.
A primary motivation for developing methods suitable for CSH C18 is to take advantage of its unique loadability. To highlight the utility of an optimized trap-elute CSH C18 separation, we investigated loading more sample in a nanoLC-MS analysis. This can be important when needing to extend the dynamic range of an analysis. Researchers may want to study a low abundance protein or peptide in the midst of a larger population of proteins and peptides: for example, phosphopeptides in a cellular or plasma matrix. In order to detect and quantify the very minimally expressed phosphopeptides, a large amount of the starting material must be loaded. It has been found that CSH C18 can be used to obtain a comprehensive nanoLC analysis of such samples, because it is capable of producing high peak capacities even at relatively high mass loads.
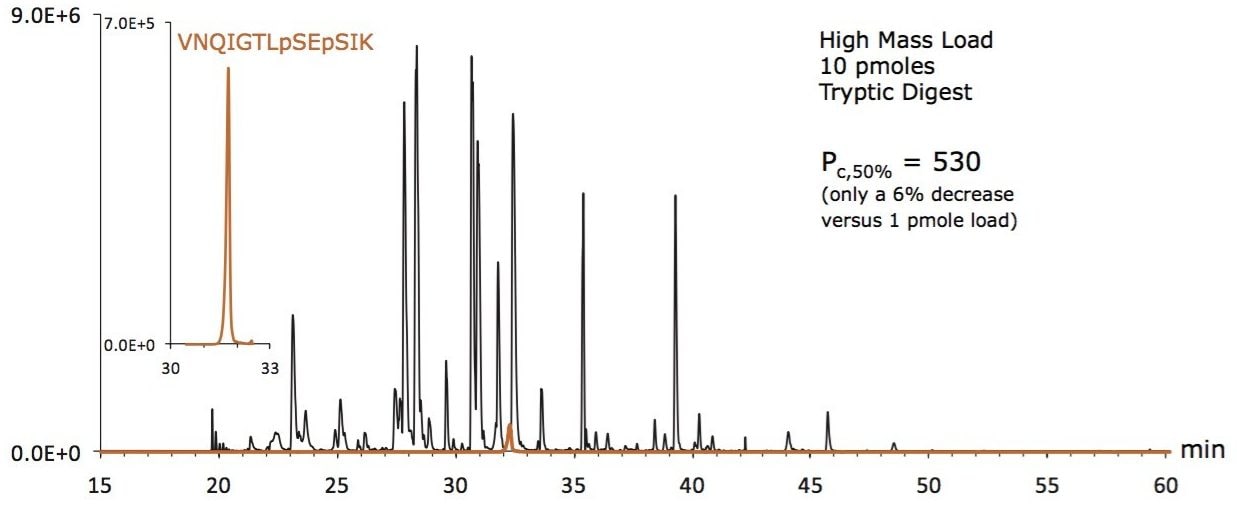
A base peak intensity (BPI) chromatogram obtained for a 10 picomole (pmole) load of an enolase tryptic digest, containing phosphopeptides illustrates the use of CSH C18 in high mass load applications (Figure 7).
It is apparent that this 10 pmole load of the enolase digest yields a comprehensive representation of the different peptides in the mixture, including the diphosphorylated species, VNQIGTLpSEpSIK. The inset extracted ion chromatogram for VNQIGTLpSEpSIK on Figure 7 shows that signal-to-noise for this peptide is remarkably high. Moreover, the distribution of signals seen in Figure 7 correlate well to data obtained for this sample when using narrow bore (2.1 mm I.D.) column chromatography.15 And since CSH C18 was employed in this nanoLC analysis, peak capacity decreased by only 6% when the mass load was increased from 1 pmole (Figure 6) to 10 pmoles, as shown in Figure 7.
The presented work defines the considerations that must be made in order to optimize trap-elute peptide separations, including the retentivity of stationary phases and the configuration with which a trap is paired with an analytical column. By employing the dual heating capabilities possible with the combined use of an ACQUITY UPLC M-Class System and a CH-A heater, we have developed high peak capacity trap-elute nanoLC peptide separations using a novel CSH C18 analytical column. This configuration has been optimized for use with a Symmetry C18 trapping column by adjusting column temperatures to affect phase retentivity as needed to maximize re-focusing of peptide analytes on the analytical column. Along with a two pump reverse-flush, trap-elute configuration, very high peak capacities can be reliably obtained. More importantly, this method development ensures that the unique attributes of the CSH C18 stationary phase, such as its loadability, can be readily exploited. Optimized, high sample load nanoscale trap-elute peptide separations with CSH C18 can be used to extend the dynamic range of an analysis and to facilitate detection of low abundance species.
720005047, May 2014