
This application note demonstrates how the purity of chlorogenic acid which is isolated from Aronia berries can be maximized by focusing the gradient to improve resolution of the compound of interest.
Compounds and extracts isolated from natural products such as the Aronia berry (Aronia Melanocarpa), have found uses in medicine, agriculture, cosmetics and food in ancient and modern societies around the world.1 The ability to access and purify these compounds through chromatographic separation has been a major driving force in research and discovery. Chromatographic methods can be developed on any scale, but when developed at a small scale and transferred to a larger scale, valuable sample material and solvents are conserved.
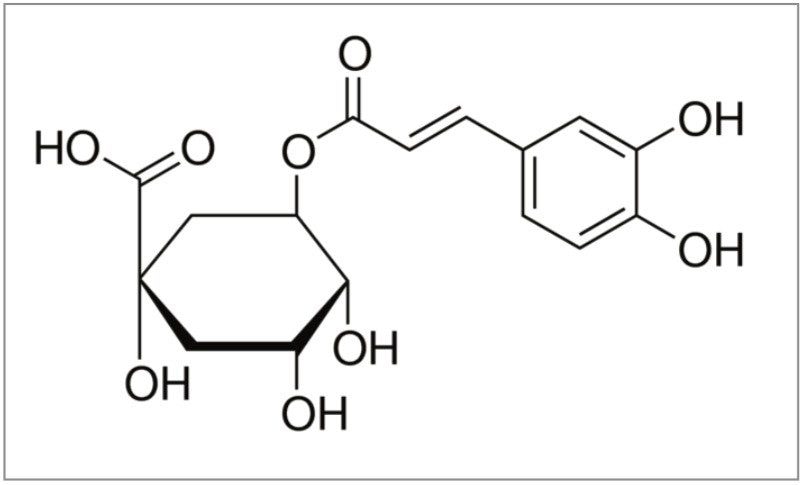
In this application note, the antioxidant chlorogenic acid (Figure 1) is purified from Aronia berries. The isolation is used to demonstrate method development, scale-up, and fraction collection using the Waters Prep 150 LC System. The application also demonstrates how purity of the isolate can be maximized by focusing the gradient to improve resolution of the compound of interest.
|
Analytical column: |
SunFire C18, 5 μm 4.6 x 50 mm |
|
Analytical flow rate: |
1.46 mL/min |
|
Prep column: |
SunFire C18 OBD Prep, 19 x 50 mm, 5 μm |
|
Prep flow rate: |
24.9 mL/min |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Detection: |
2489 UV/V is at UV 324 nm (Autopurification flow cell) |
|
Temperature: |
Ambient |
|
Pump: |
2545 Binary Gradient Module |
|
Injector: |
FlexInject Manual Dual Injection Module configured with 100 μL loop (analytical) and 2000 μL loop (prep) |
|
Collector: |
Waters Fraction Collector III |
|
Data collection: |
ChromScope v1.6 |
Prep Chromatography System
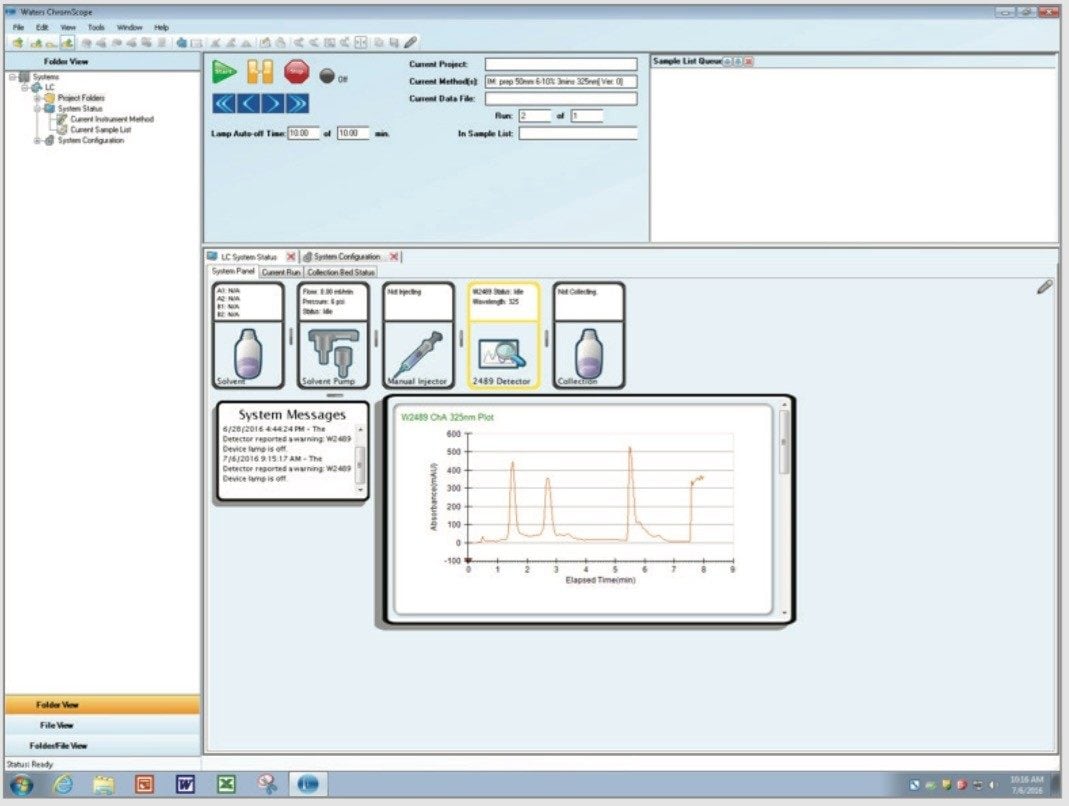
The Waters Prep 150 LC System (Figure 2) consisted of the 2545 Binary Gradient Module, a solvent delivery module capable of flow rates up to 150 mL/min; the 2489 UV/Visible Detector; the Waters Fraction Collector III; and the FlexInject Manual Dual Injector Module controlled by ChromScope v1.6, which manages UV-based collection triggers and tracks samples, fractions, and associated data through its straightforward browser (Figure 3). The system configuration is an entry level, easy-to-use modular design that is capable of purifying several samples daily.

Fresh Aronia berries were obtained from an organic farm and dried through lyophilization. The dried berries were ground into a fine powder and subsequently 1.5 g reconstituted with 3.0 mL acidified water (1 M HCl). The sample solution was mixed well, centrifuged to remove solids, and the supernatant filtered through a 0.45 μm syringe filter.
A chlorogenic acid reference standard obtained from Sigma-Aldrich, part # C3878, was prepared by dissolving 5.0 mg in 20 mL acidified water (1 M HCl). The reference standard was used to confirm retention time of the chlorogenic acid isolated product.
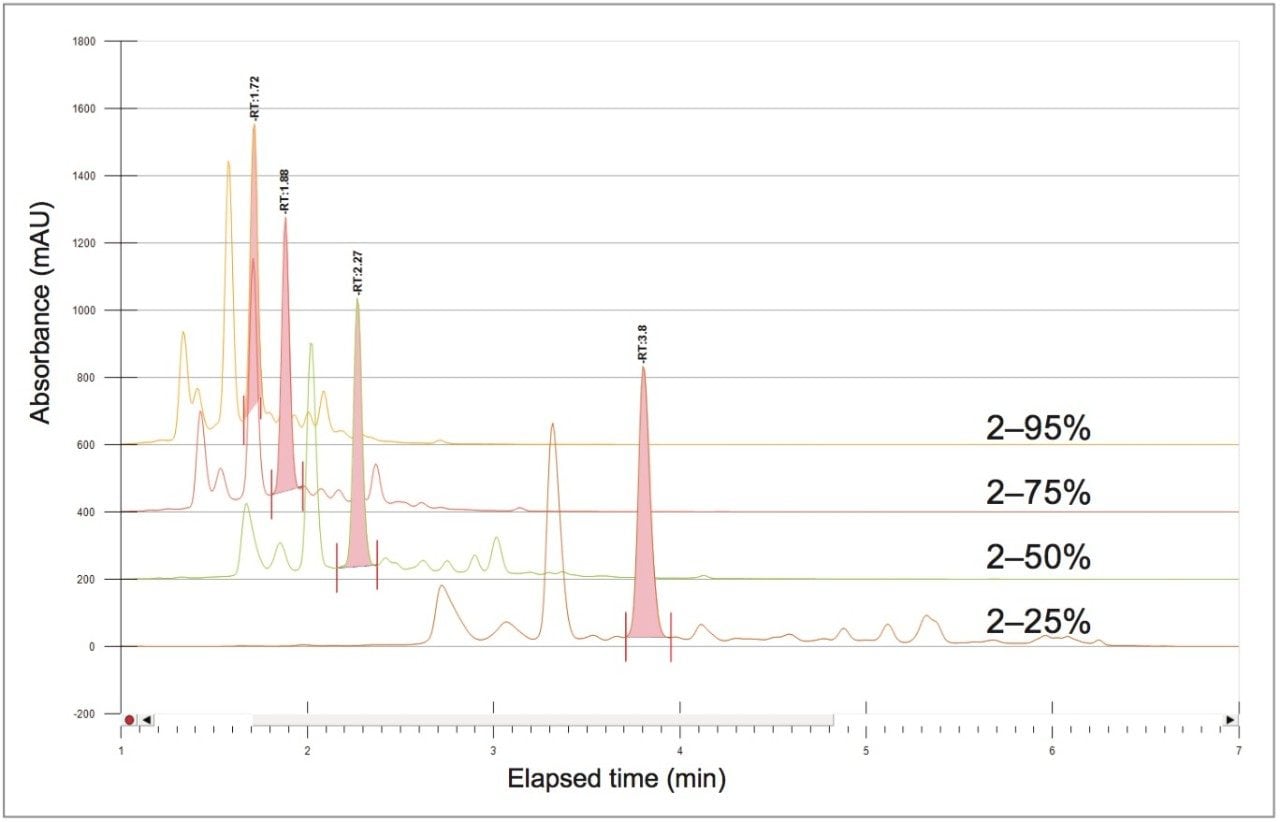
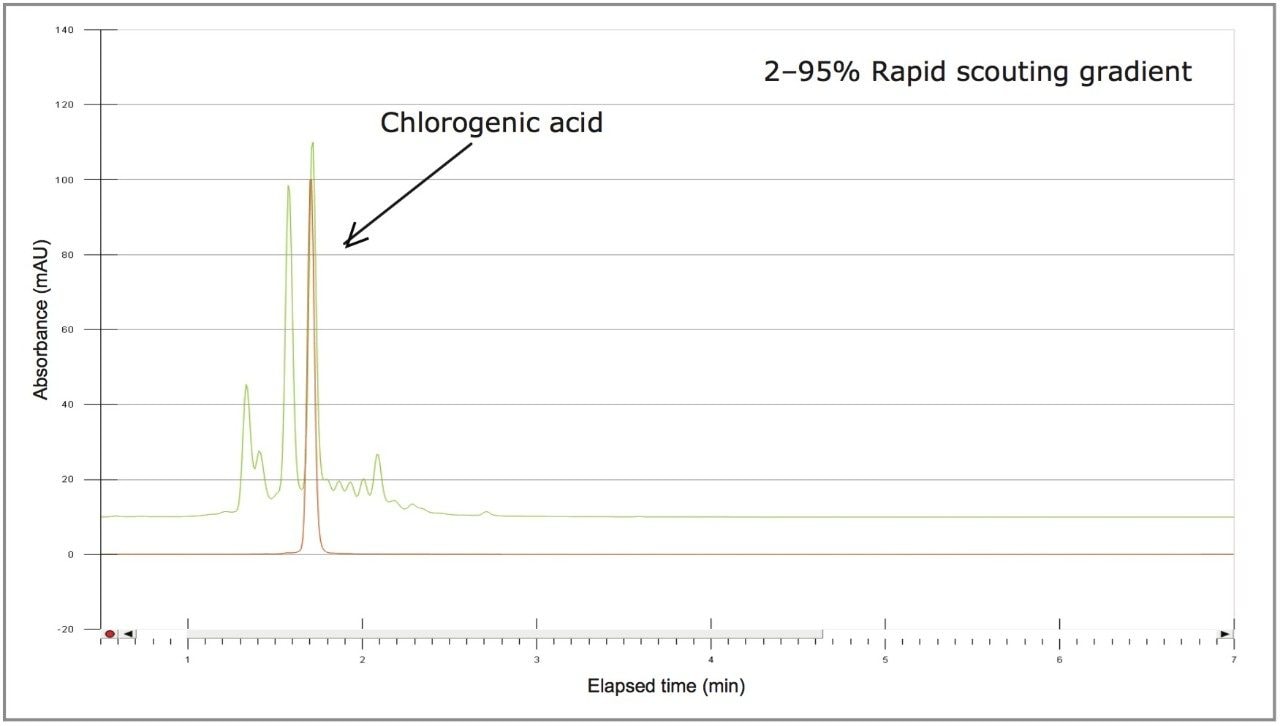
The sample solution was analyzed at the analytical scale with four rapid scouting gradients. Each gradient started from a 2% organic concentration and rapidly increased linearly over 5 minutes to a 25%, 50%, 75%, or 95% organic concentration Figure 4). The scouting run from 2–25% provided the best resolution of chlorogenic acid and surrounding impurities, as determined by a reference standard injection.
With most separations, to improve the resolution between the compounds of interest and close eluting impurities, a focused gradient can be used. The focused gradient typically begins at the initial organic concentration used in the scouting run and quickly ramps to an organic concentration just before the percentage of solvent that elutes the compound of interest. Then a shallow linear gradient is performed to elute the peak of interest, and finally the column is washed quickly with a high organic concentration.
Scouting run 4 provided the highest resolution between chlorogenic acid and the impurities therefore it was used to develop the focused gradient.
The following calculations were used to generate the focused gradient.
Column volume was calculated with the equation Π x (r)2 x Length, with compensation for the volume occupied by packing material (66% as per the “Waters Optimum Bed Density (OBD) Prep Calculator”).
Time to detector =1.497 mL /1.46 mL/min Time to detector = 1.03 min
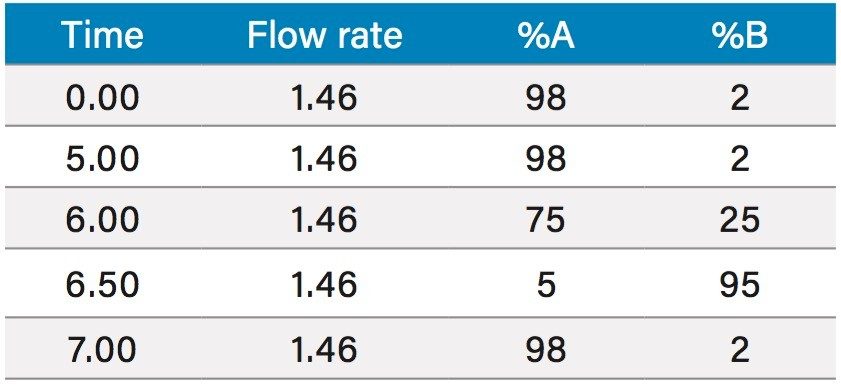
Based upon the calculations, a focused gradient was developed to begin at the initial conditions of the scouting run (2%) with a quick ramp to the beginning concentration of the focused gradient (10%). The solvent concentration increased linearly for 8.7 minutes to elute chlorogenic acid, and finally the column was washed with high organic. Adequate resolution between chlorogenic acid (3.8 mins) and surrounding impurities was obtained by the focused gradient (Table 2, Figure 5).
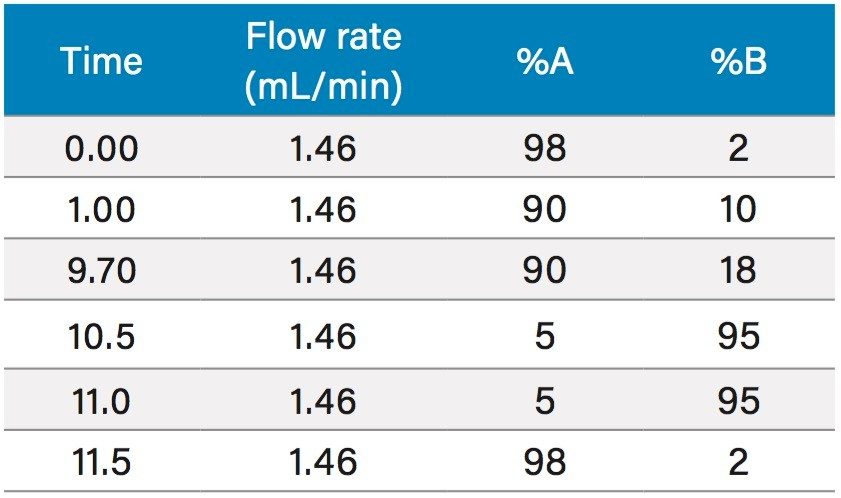
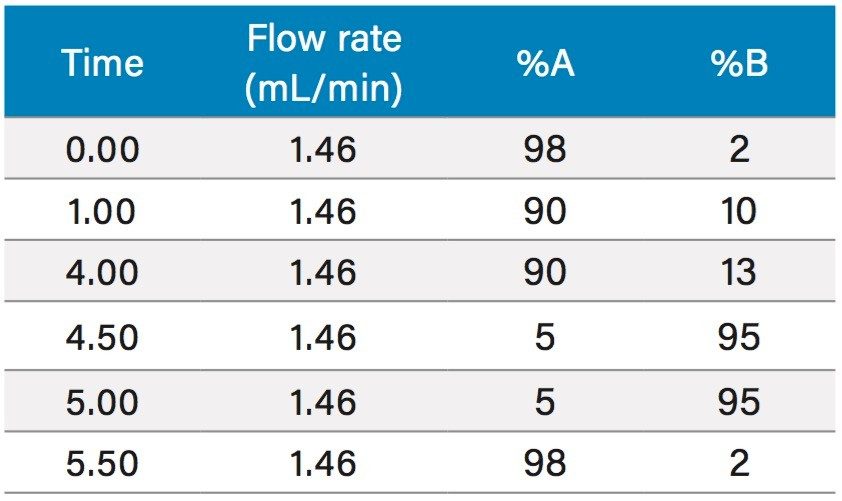
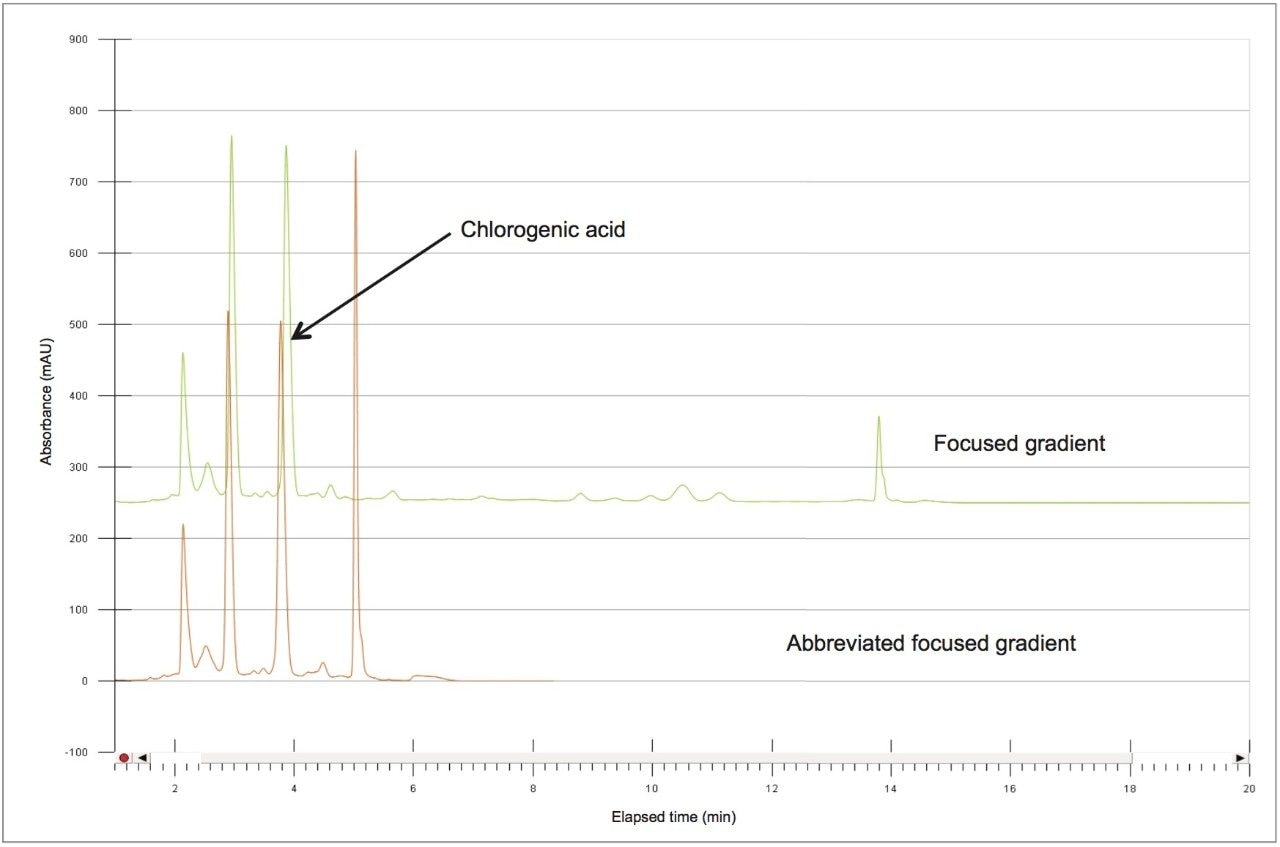
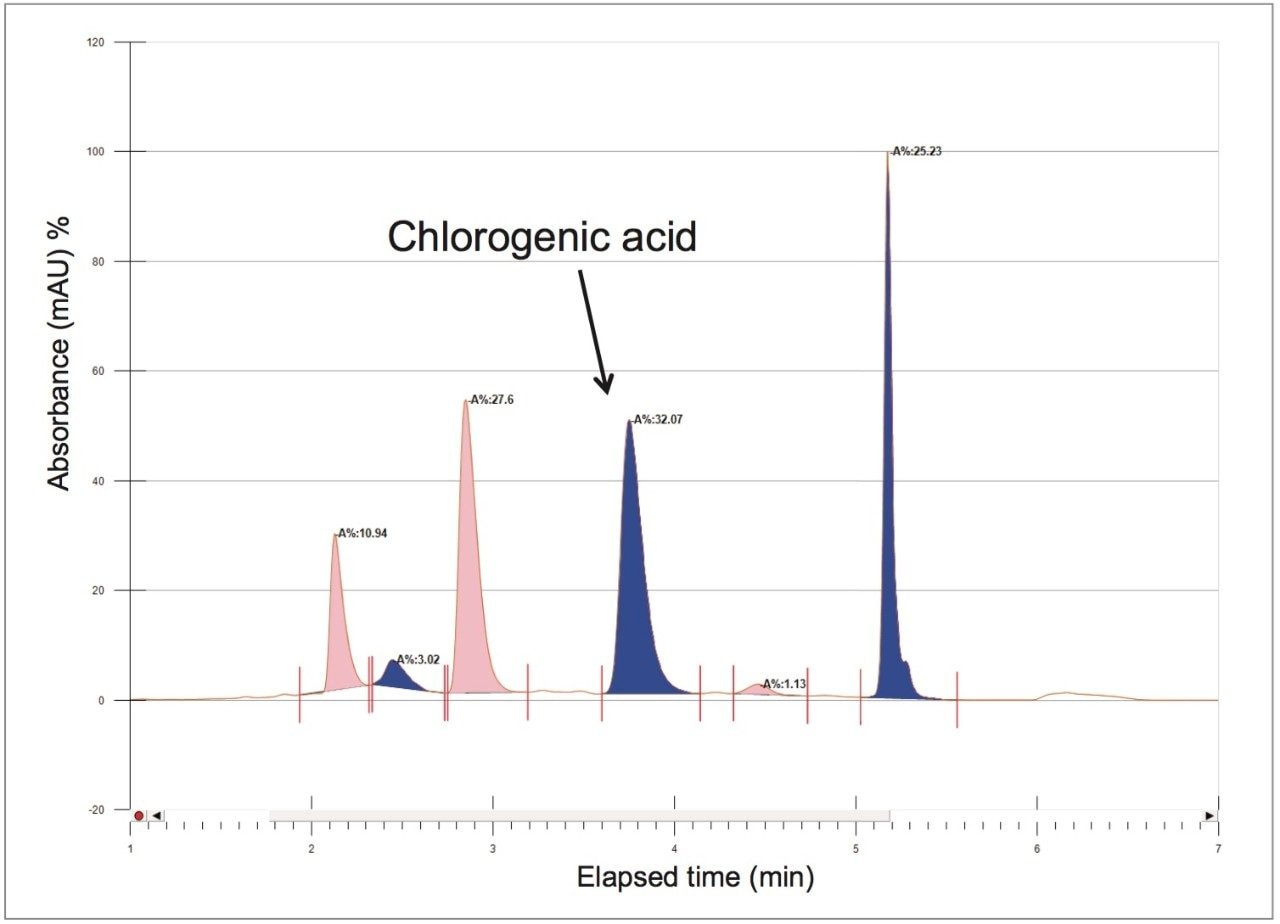
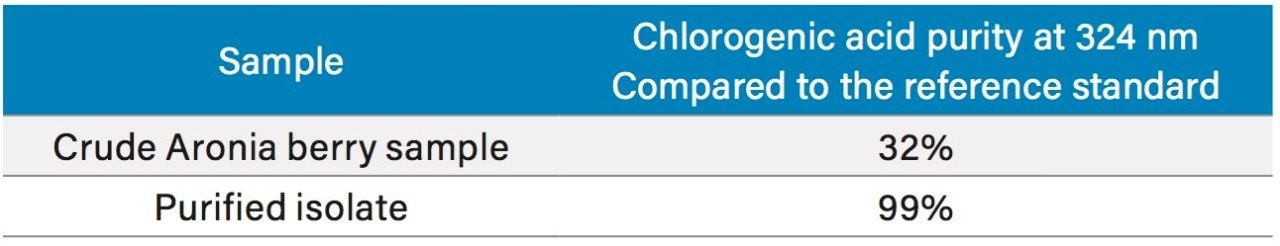
To increase throughput and reduce run time by approximately 6 minutes, the column was washed with solvent immediately after the elution of chlorogenic acid, rather than executing the entire focused gradient (Figure 5). The purity of chlorogenic acid in the crude berry sample was determined by the ChromScope Software to be 32% by area calculation when separated by the abbreviated focused gradient (Figure 6).
To increase throughput, the abbreviated focused gradient was scaled-up from analytical to prep. The following calculations were used to determine the appropriate flow rate and injection volume for the 19 mm I.D. prep column. The calculations were based upon the flow rate and injection volume used for the 4.6 mm I.D. analytical column:
Flow Prep = Flow Analytical x (Diameter Prep/Diameter Analytical)2
Flow Prep = 1.46 mL/min x (19 mm/4.6 mm)2
Flow Prep = 24.9 mL/min
Volume Prep = Volume Analytical x (Diameter Prep /Diameter Analytical)2 x (Length Prep /Length Analytical)
Volume Prep = 100 μL x (19 mm/4.6 mm)2 x (50 mm/50 mm)
Volume Prep = 1706 μL
The 4.6 mm I.D. column flow rate of 1.46 mL/min and 100 μL injection volume scales-up to a 24.9 mL/min flow rate and 1706 μL injection volume for the prep column. Retention time was identical on both columns after scale-up using the abbreviated focused gradient (Figure 7).
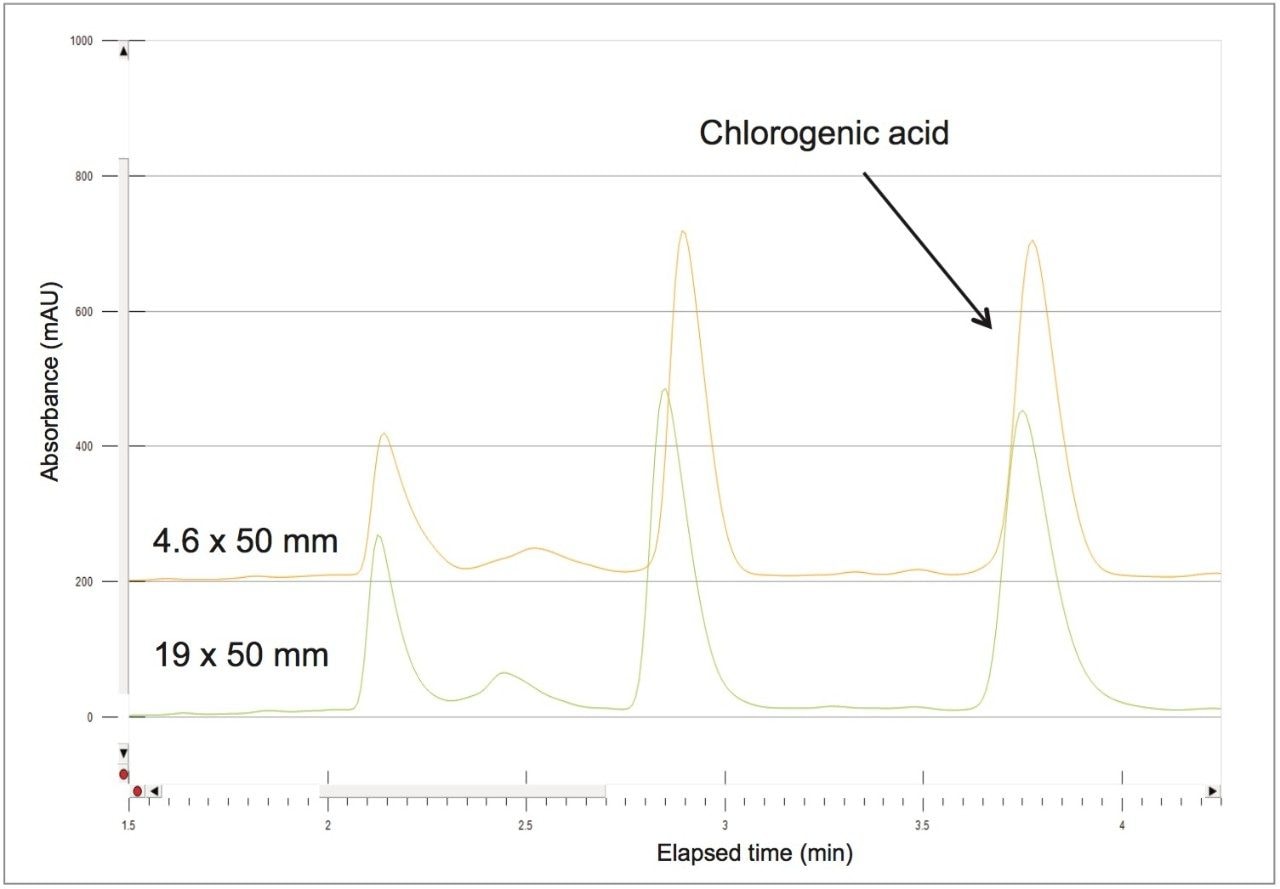
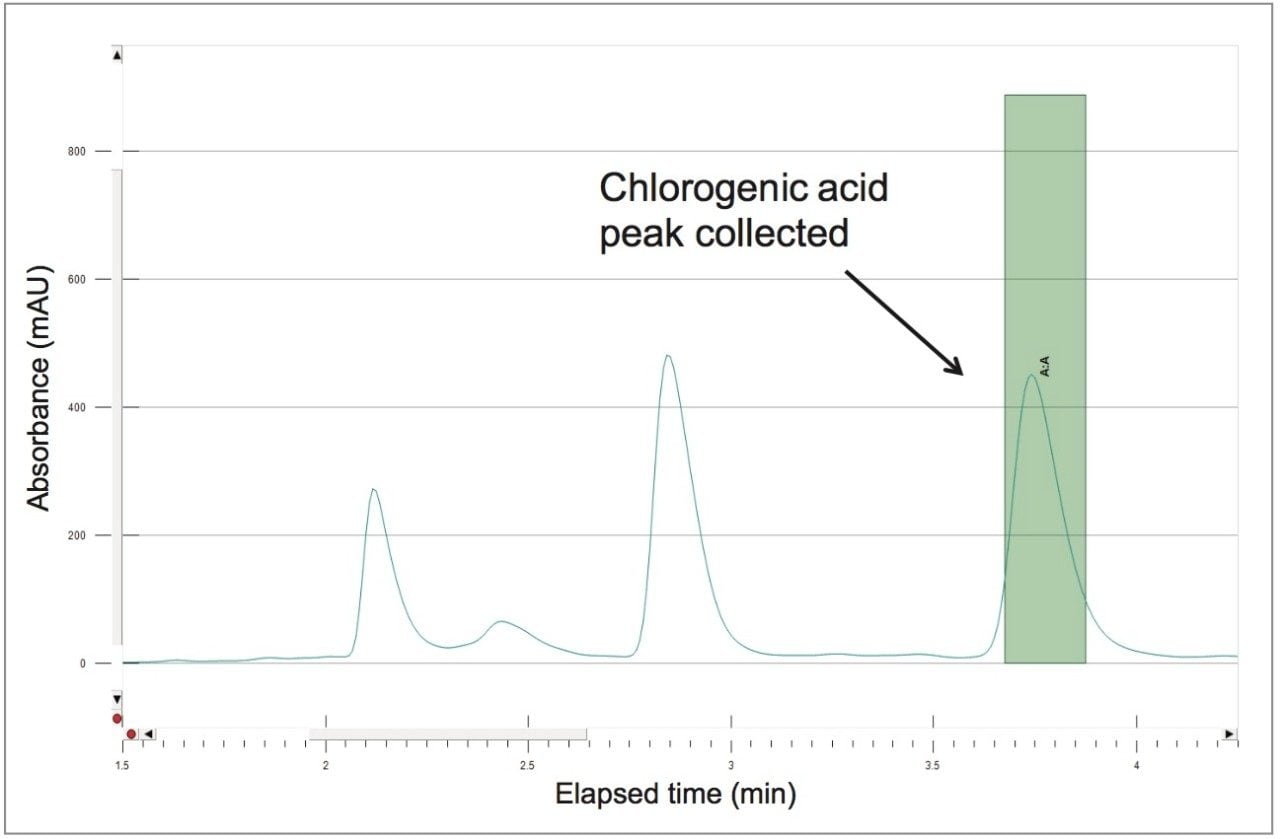
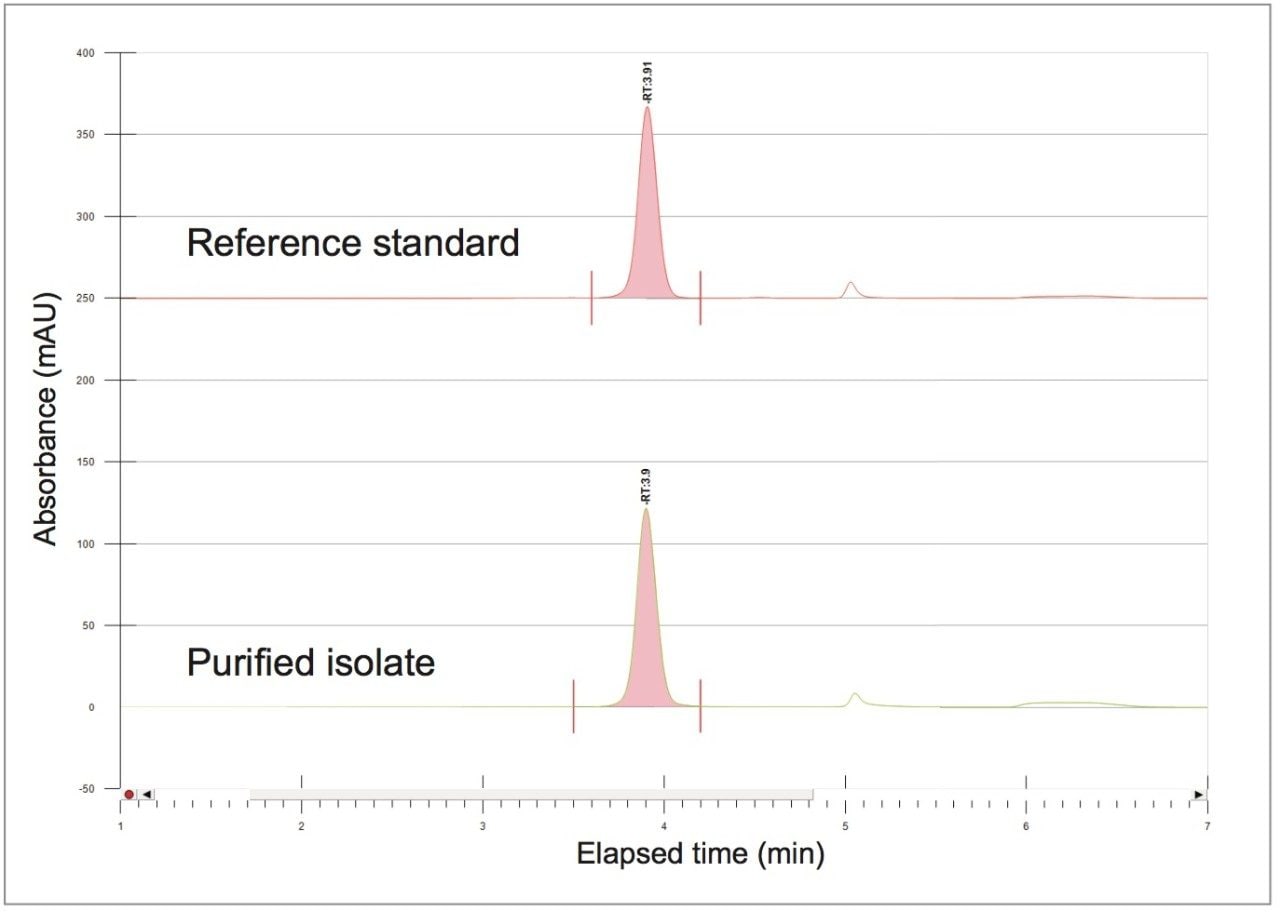
A collection method using a μ absorbance threshold trigger was developed to collect the chlorogenic acid fraction at prep scale using the ChromScope fraction collection simulator. After collection (Figure 8), the retention time and purity of the fraction was compared to the reference standard (Figure 9, Table 4). Purity of chlorogenic acid increased from 32% in crude Aronia berries to 99% when compared to the chlorogenic acid reference standard at 324 nm. The isolate was also analyzed by the scouting gradient to demonstrate the increase in purity compared with the crude Aronia berry sample (Figure 10).
Here, fast and efficient isolation of the antioxidant chlorogenic acid from crude Aronia berries was demonstrated. Throughput was maximized by first generating a focused gradient, then optimizing the focused gradient for run time. The purification method effectively yielded a chlorogenic acid isolate comparable in purity to the reference standard at 324 nm. The entire purification when scaled from analytical to prep, was successfully accomplished using the Waters Prep 150 LC System.
720005811, September 2016