In this application note a microflow method was developed for the quantitation of fluticasone propionate in human plasma using solid phase extraction followed by reversed phase chromatography and tandem quadrupole mass spectrometry. The use of the ionKey/MS System in the trapping configuration provides the required sensitivity and robustness for the fluticasone propionate assay.
The research method was evaluated for inter- and intra-batch precision and accuracy, matrix factor, recovery, selectivity and carryover, with validation based on the FDA “Guidance for Industry” for Bioanalytical methods.3,4 A full validation study across three batches was undertaken for rat plasma. Partial/cross validation of single batches, including inter-batch precision and accuracy, specificity, matrix effects, and recovery were completed in mouse and human plasma.
In the case of linearity, the standard curves for all of the analytes gave r2 values above 0.99 across the three batches of the intra-batch validation. Single batch validation of mouse and human plasma showed comparable r2 values.
Fluticasone propionate (Figure 1) is a glucocorticoid indicated for the prophylactic treatment of asthma. It is administered via inhalation from an aerosol-type of device or powder inhaler. Due to its low administered dose and the corresponding low circulatory levels, it becomes necessary to have an extraordinarily sensitive bioanalytical assay to correctly define the pharmacokinetics in plasma (<10 pg/mL).1
To obtain the required sensitivity needed for this assay, microflow LC-MS/MS was utilized with the ionKey/MS System. Additionally, the use of multidimensional chromatography, specifically a trap and elute strategy, provided further sample cleanup and facilitated the loading of larger injection volumes. The ability to inject sample volumes typical for analytical scale LC-MS analysis on the iKey Separation Device can provide substantial gains in sensitivity.
Using this technique, we were able to demonstrate a lower limit of quantification (LLOQ) of 0.48 pg/mL for fluticasone propionate in human plasma. This enhanced level of sensitivity allows for the accurate determination of the pharmacokinetics of fluticasone in plasma.
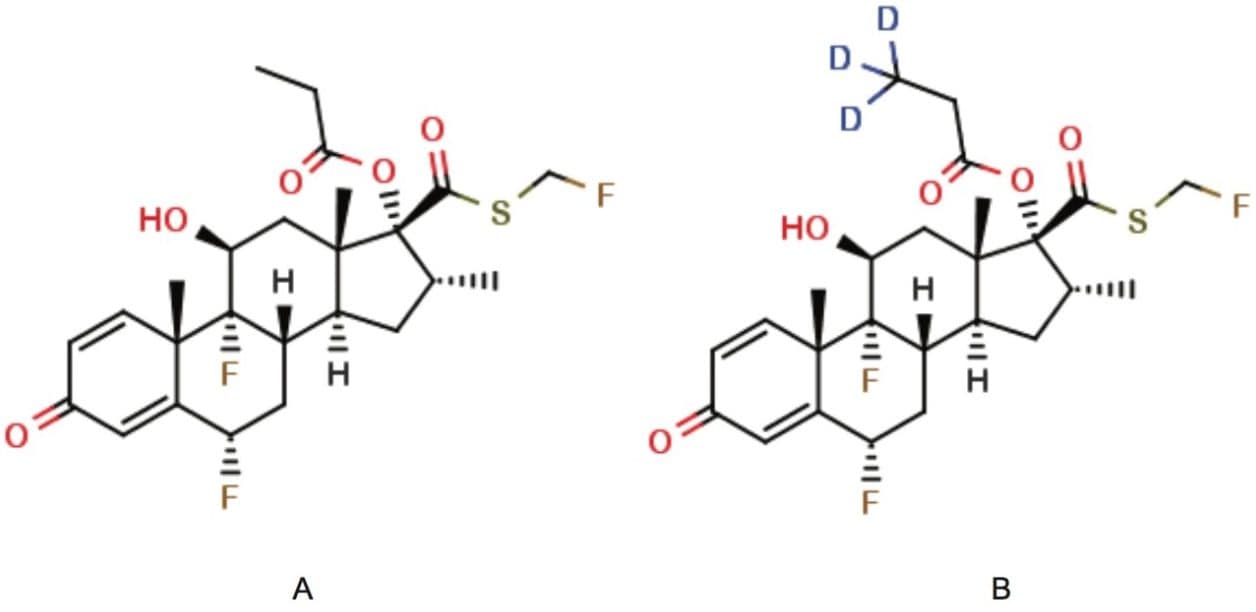
Figure 1. Fluticasone propionate and d3-fluticasone propionate.
(A) Fluticasone propionate; molecular formula: C25H31F3O5S; MW: 500.57
(B) d3-Fluticasone propionate; molecular formula: C25H28D3F3O5S; MW: 503.59.
Fluticasone propionate standard solutions and QC solutions in the range from 2.44 to 2,500 pg/mL were prepared in 25% methanol. Plasma standards (0.244–250 pg/mL) and QC samples (1.5, 10, and 100 pg/mL) were prepared by spiking 60 µL of fluticasone propionate standard aqueous solutions into 520 µL of human plasma. Then, 20 µL of internal standard solution (1 ng/mL fluticasone propionate-d3 in human plasma) was added. A solution of 0.2 M zinc sulfate (700 µL) was used for protein precipitation. Samples were vortexed for 5 minutes and then centrifuged for 5 min at a speed of 8000 rpm. The supernatant (1 mL) was transferred to a preconditioned Sep-Pak C18 plate. The plate was preconditioned with 0.8 mL of methanol and 0.8 mL water. The loaded plate was washed with 1.6 mL of water and 0.8 mL of 20% methanol in water. Both analyte and internal standard were then eluted by using 300 µL of 90% acetonitrile in water. The eluted samples were evaporated to dryness and then reconstituted in 100 µL of 25% methanol.
|
LC system: |
ACQUITY UPLC M-Class System, configured with optional trap and back flush elution |
|
Separation device: |
iKey UPLC HSS T3 1.8 μm 130A, 150 μm x 50 mm |
|
Trap column: |
300 μm x 50 mm Symmetry C18 |
|
Mobile phase A: |
10 mM Ammonium Bicarbonate buffer pH 7.7 |
|
Mobile phase B: |
90:10 Methanol:Isopropanol |
|
Loading solvent: |
99:1 mobile phase A:B, 20 μL/min for 3 min |
|
Weak needle wash: |
10 mM Ammonium bicarbonate Buffer pH 7.7 |
|
Strong needle wash: |
90:10 Methanol:isopropanol |
|
Seal wash: |
80:20 Water:acetonitrile |
|
Elution flow rate: |
3.0 μL/min |
|
Column temp.: |
50 °C |
|
Injection volume: |
20 μL |
During method development, it was determined that ammonium bicarbonate buffer (pH=7) gave the greatest sensitivity. However, contamination is a concern when operating in neutral and basic pH enviroments due to the accumulation of bacteria and particules debris. An unique protocol for preconditioning the LC system was developed, which allowed us to operate robustly in this neutral pH range.2
|
Time (min) |
Flow (μL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
3 |
60 |
40 |
Initial |
|
6 |
3 |
5 |
95 |
6 |
|
8 |
3 |
5 |
95 |
6 |
|
9 |
3 |
60 |
40 |
6 |
|
12 |
3 |
60 |
40 |
6 |
|
MS system: |
Waters ionKey/MS |
|
Ionization mode: |
ESI + |
|
MS/MS transitions: |
Fluticasone propionate 501.3>313.2 d3-Fluticasone propionate 504.3>313.2 |
|
Capillary voltage: |
3.7 kV |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
150 L/hr |
|
Cone voltage: |
28 V |
|
Collision energy: |
16 V |
|
Data Management: |
MassLynx 4.1; TargetLynx |
The LC-MS analysis of fluticasone propionate and its stable labeled isotope d3-fluticasone propionate was performed on an ionKey/MS System comprised of an ACQUITY UPLC M-Class System coupled with a Xevo TQ-S Mass Spectrometer in MRM mode. The signature attributes of this system include high sensitivity, solvent savings, and ease of use.
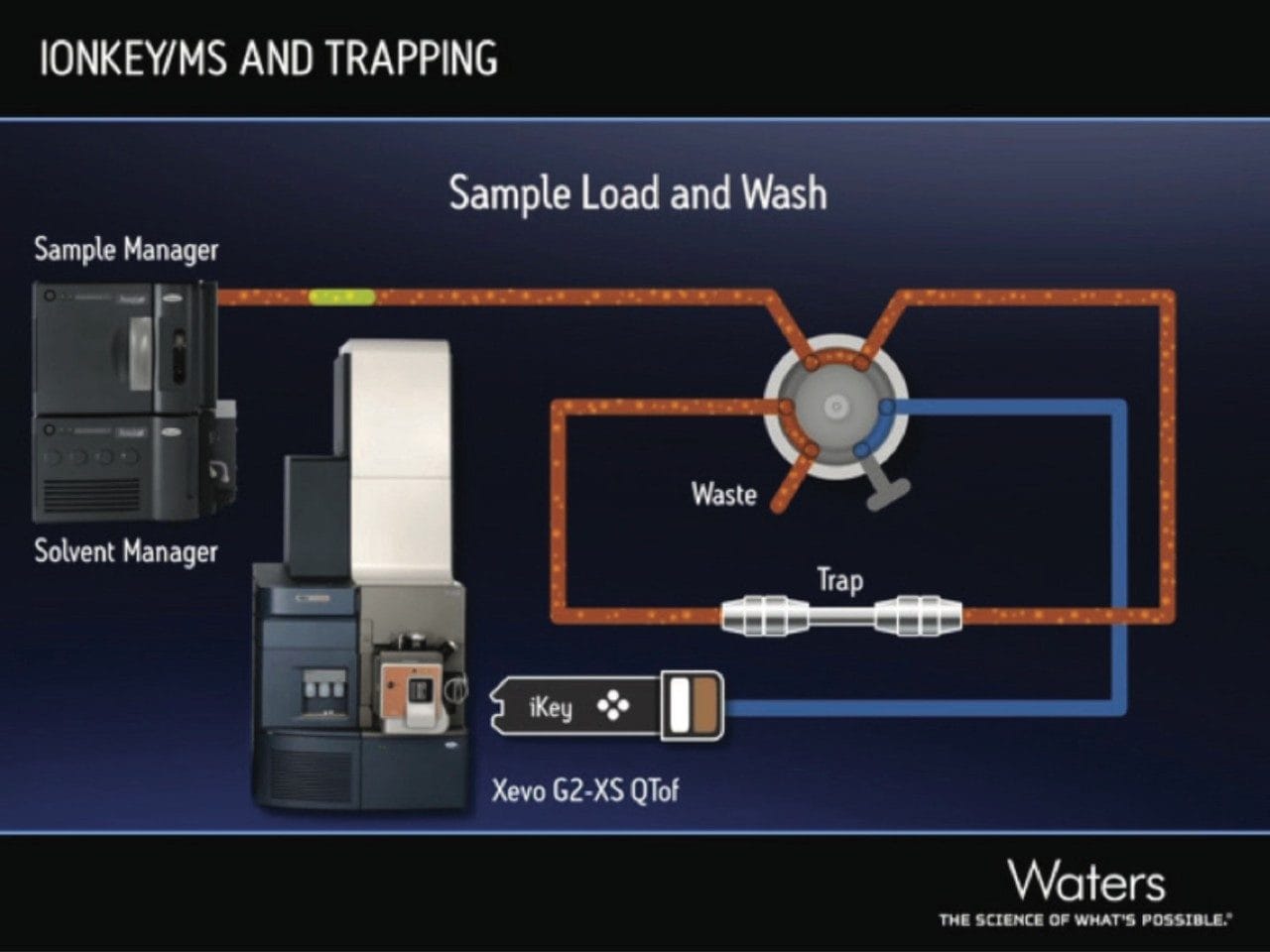
The separation was achieved by using a 2D trap-and-elute configuration, as illustrated in Figure 2. This configuration offers a number of advantages, including: improved focusing and retention of hydrophilic compounds, increased sample loading, reduced duty cycle times compared to direct injection methodologies, and automated sample cleanup and downstream protection of the iKey Separation Device.3 These advantages improve the limit of detection of the assay and the robustness of the system.
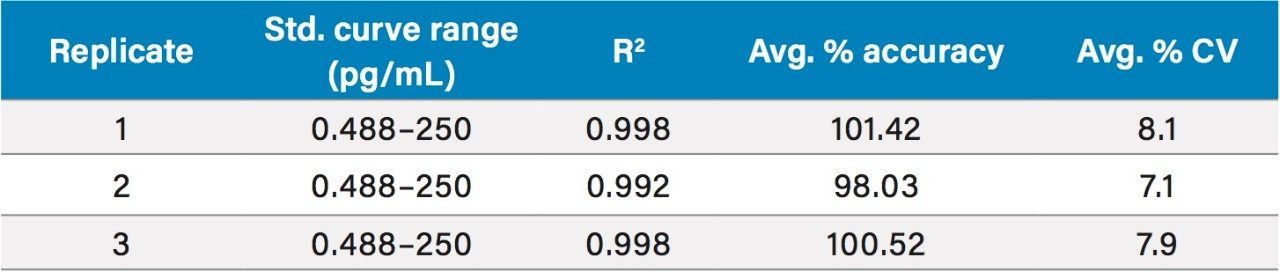
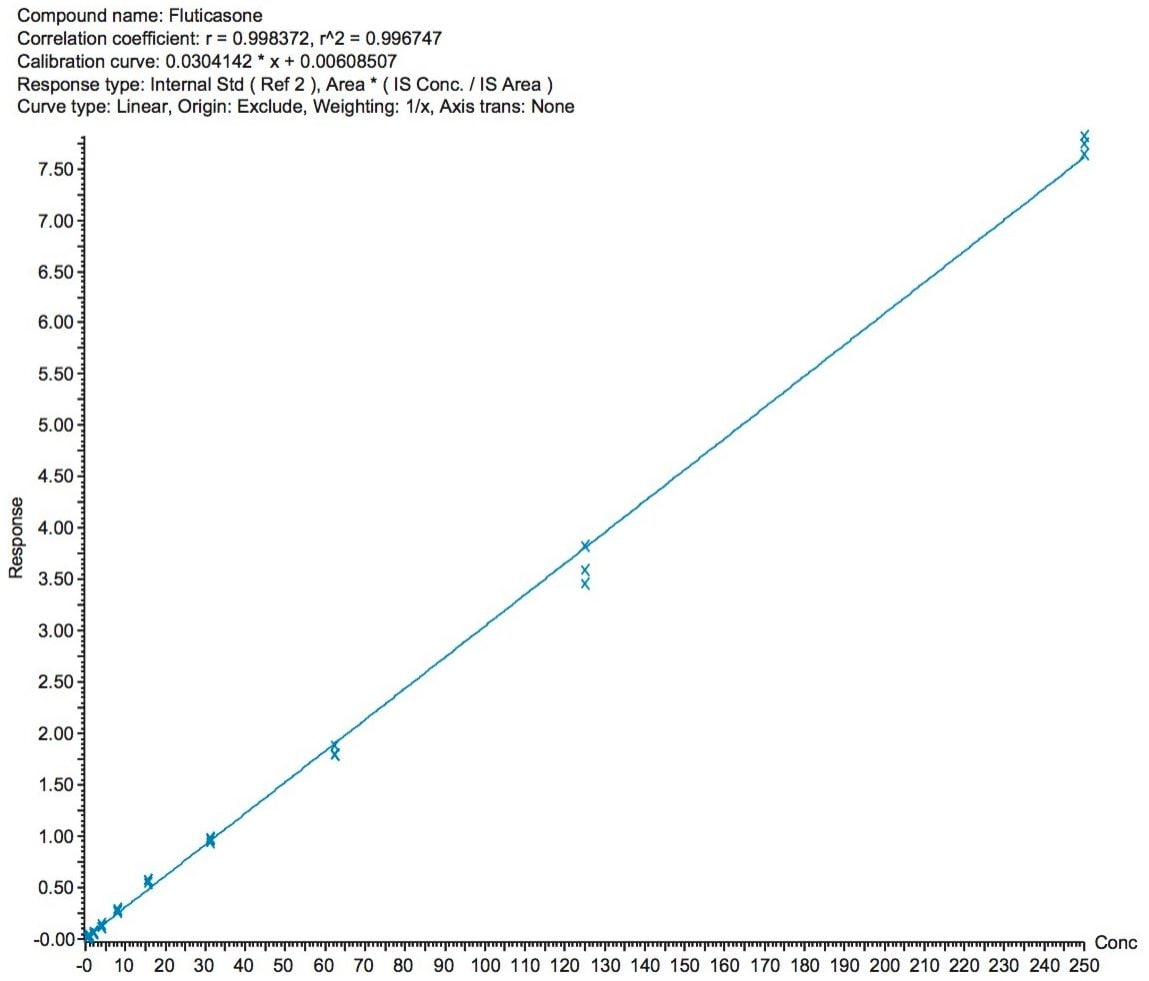
The fluticasone propionate human plasma calibration curve was validated with serial diluted solutions in human plasma ranging from 0.488 to 250 pg/mL. Quality control (QC) samples were prepared at 1.5, 10, and 100 pg/mL. QC samples at each level were prepared in duplicate.
Peak area ratios (PARs) of the analyte peak area to the IS peak area were calculated. The calibration curves were constructed using PARs of the calibration standards by applying a (1/x) weighted linear regression model. All standard curves from three validation runs had coefficients of determination (r2) >0.99. A summary of standard curve performance for fluticasone propionate is shown in Table 1. Representative standard calibration curve is shown in Figure 3.
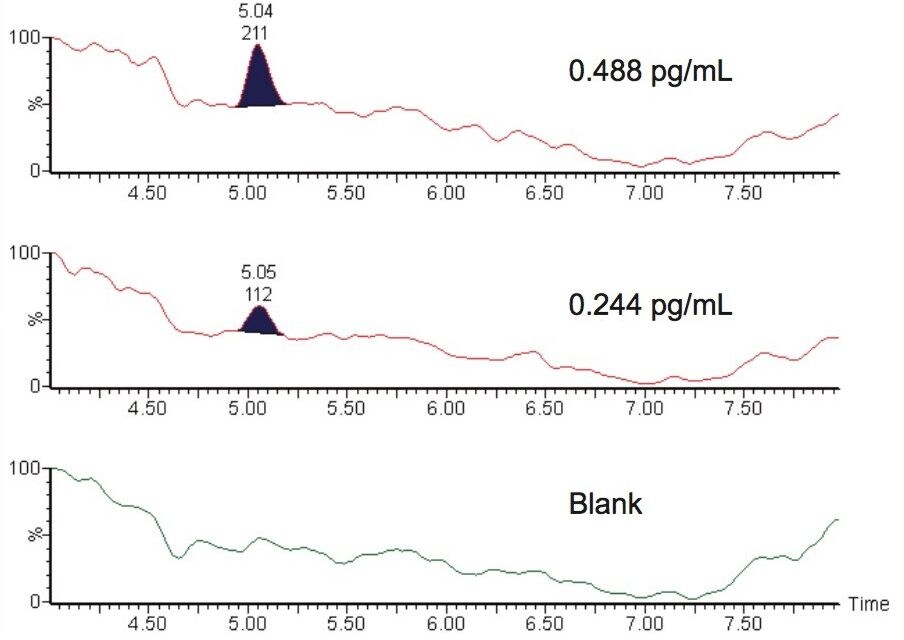
The overall assay sensitivity was determined to be 0.244 pg/mL for the limit of detection (LOD), and 0.488 pg/mL for the limit of quantitation (LLOQ), Figure 4.
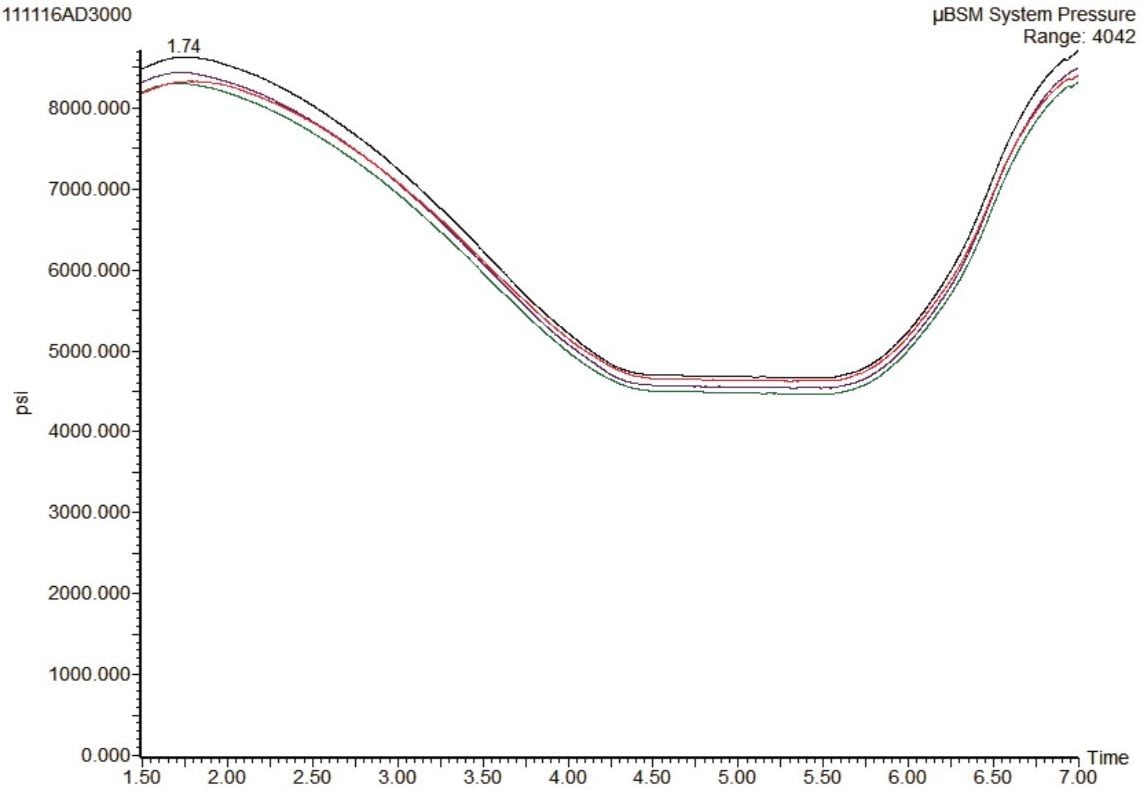
Robust and reliable data are essential for bioanalysis. However, plugging of the small scale chromatographic components by the relative dirtiness of the biological samples is a major concern when implementing microflow chromatography in routine analysis in a bioanalytical laboratory.
The robustness of this microflow system was tested by injecting the same sample over 3000 times. The system pressure traces from this study are shown in Figure 5. No significant increase in system pressure was observed, indicating that none of the frits, tubing, or connecting fittings have been blocked over the course of the study.
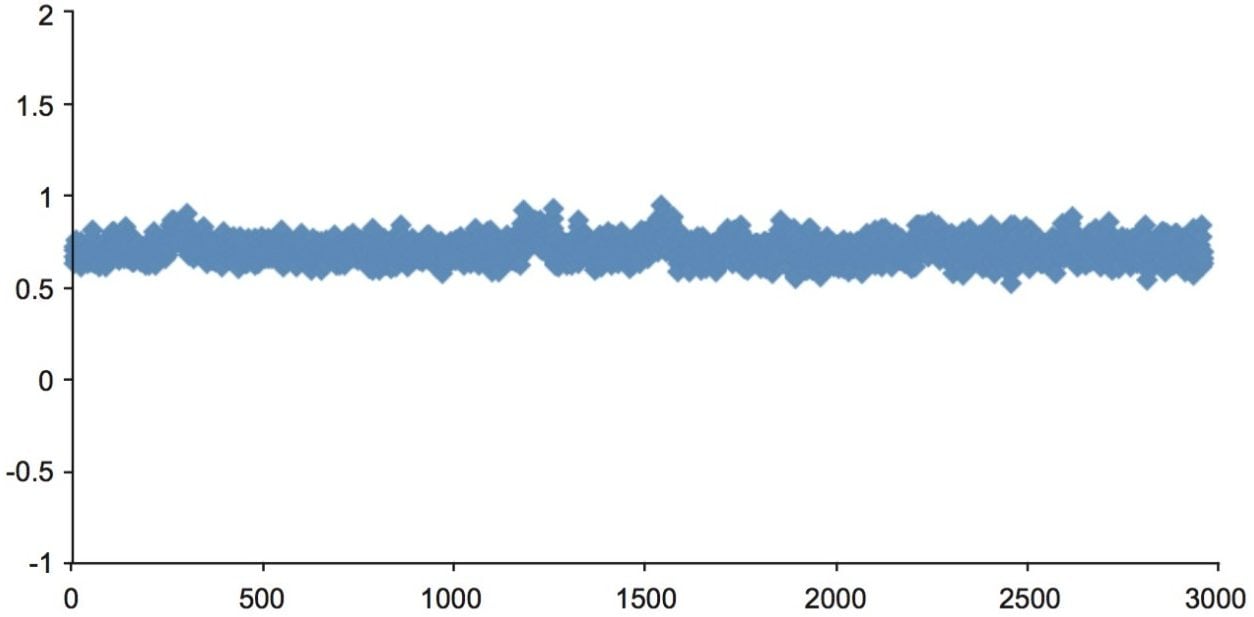
The peak area ratios of the analyte area peak to internal standard peak demonstrate good reproducibility as shown in figure 6. Percent RSD was 8% for 3000 injections.
A microflow method was developed for the quantitation of fluticasone propionate in human plasma using solid phase extraction followed by reversed phase chromatography and tandem quadrupole mass spectrometry. The use of the ionKey/MS System in the trapping configuration provides the required sensitivity and robustness for the fluticasone propionate assay.
720005961, March 2017