The objective of this application note is to demonstrate a method for improving the recovery of intact antibodies from reversed-phase liquid chromatography (RPLC) performed at relatively low column temperatures.
Method for improving the recovery of intact antibodies from reversed-phase liquid chromatography (RPLC) performed at relatively low column temperatures
The market for monoclonal antibodies (mAbs) has grown rapidly in the past 30 years, partially due to their high efficacy and specificity for the treatment of various diseases with limited side effects.1 However, the large size of an antibody along with its manufacturing process results in significant structural complexity and heterogeneity that calls for comprehensive characterization to ensure drug safety. Compared to other separation techniques employed in the characterization of biomolecules, reversed-phase liquid chromatography (RPLC) is often used for its high separation efficiency and flexibility to interrogate proteins at different molecular levels, including intact, subunit, and peptide analysis.2,3 Unfortunately, conventional RPLC separations of intact antibodies are commonly impaired by restricted diffusion from porous-based, small pore size particles, as well as adsorption and secondary interaction between stationary phase and analytes caused by non-ideal surface chemistries.3 To increase the recovery and resolution of mAb separations, high column temperatures (≥80 °C) and strong ion-pairing reagents (such as TFA) are commonly used, though they pose a risk of causing on-column sample degradation and reduced column life. To this end, it is advantageous to develop LC methods that incorporate a column technology tailored to antibody separations so that it might be possible to preserve sample integrity, extend column life, and more accurately characterize and monitor critical quality attributes.
The BioResolve RP mAb Polyphenyl Column was designed for the separation of macromolecules to address the challenges listed above. For this reason, it is based on uniquely optimized silica-based, solid-core particles, and novel ligand chemistry. Compared to traditional, fully-porous silica particles, the solid core, silica-based, 2.7 µm particles are comprised of a carefully optimized porous layer coating that reduces intra particle diffusion distances and minimizes peak broadening. The polyphenyl bonded phase is comprised of rigidly constrained, sterically bulky phenyl moieties that can facilitate more discrete desorption and provide high surface coverage to reduce secondary interactions, leading to potential improvements in protein recovery. Together, these characteristics make the BioResolve RP mAb Polyphenyl Column a promising option in the development of high throughput separation of intact antibodies at lower temperatures, as it is sometimes needed to reduce the thermal induced degradation of mAbs.4,5
Accordingly, the objective of this application note is to demonstrate that, by using the BioResolve RP mAb Polyphenyl Column, high recoveries can be achieved for intact antibodies across a range of various temperatures. In this study, a highly efficient separation method was developed for resolving intact mAbs using the BioResolve RP mAb Polyphenyl Column and a leading commercially available column with porous particles and similar surface ligand chemistry. A panel of mAbs was selected to compare the performance between these two columns and to evaluate the applicability of the developed method for different samples.
Waters Intact mAb Mass Check Standard (p/n: 186006552) was used as reference material in addition to a panel of therapeutic mAbs, including Rituximab, Tocilizumab, and Bevacizumab. All samples were dissolved or diluted in water to obtain a solution at the concentration of 5 µg/µL. HPLC grade water, acetonitrile, trifluoroacetic acid (TFA), and difluoroacetic acid (DFA) were purchased from Fisher Scientific and used as received. N-butanol was purchased from Sigma-Aldrich.
|
System: |
ACQUITY UPLC H-Class Bio |
|
Detectors: |
ACQUITY TUV Detector 5 mm flow cell, λ = 280 nm |
|
Column: 1: |
BioResolve RP mAb Polyphenyl, 450Å, 2.7 μm (*average pore diameter is measured by Hg porosimetry), 2.1 × 50 mm (p/n: 176004156 that contains column and reference standards) |
|
2: Polymeric PS-DVB, 1500Å, 2.1 × 50 mm |
|
|
Column temp.: |
40–80 °C |
|
Sample vial: |
12 × 32 mm glass vial Total recovery (p/n: 600000750cv) |
|
Mobile phases: |
Water and acetonitrile |
|
MP additive: |
1, 0.1% TFA 2, 0.1% DFA and 5% butanol |
|
Mass load: |
2.5 μg |
|
Injection volume: |
0.5 μL |
|
Time (min) |
Flow rate (min) |
%A |
%B |
|---|---|---|---|
|
Initial |
0.500 |
75.0 |
25.0 |
|
10.00 |
0.500 |
55.0 |
45.0 |
|
11.00 |
0.500 |
20.0 |
80.0 |
|
11.50 |
0.500 |
20.0 |
80.0 |
|
11.51 |
0.500 |
75.0 |
25.0 |
|
15.00 |
0.500 |
75.0 |
25.0 |
|
Time (min) |
Flow rate (min) |
%A |
%B |
|---|---|---|---|
|
10.00 |
0.500 |
85.0 |
15.0 |
|
11.00 |
0.500 |
20.0 |
80.0 |
|
11.50 |
0.500 |
20.0 |
80.0 |
|
11.51 |
0.500 |
85.0 |
15.0 |
|
15.00 |
0.500 |
85.0 |
15.0 |
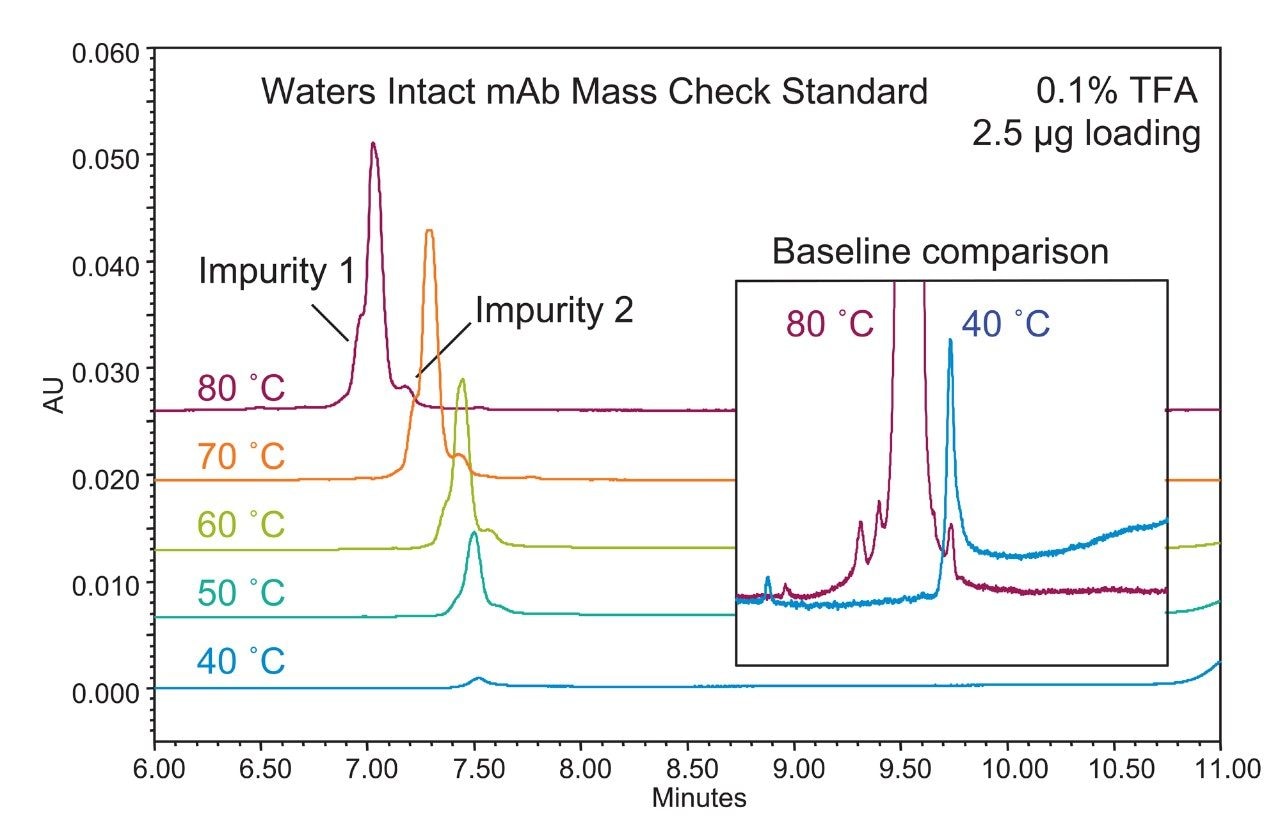
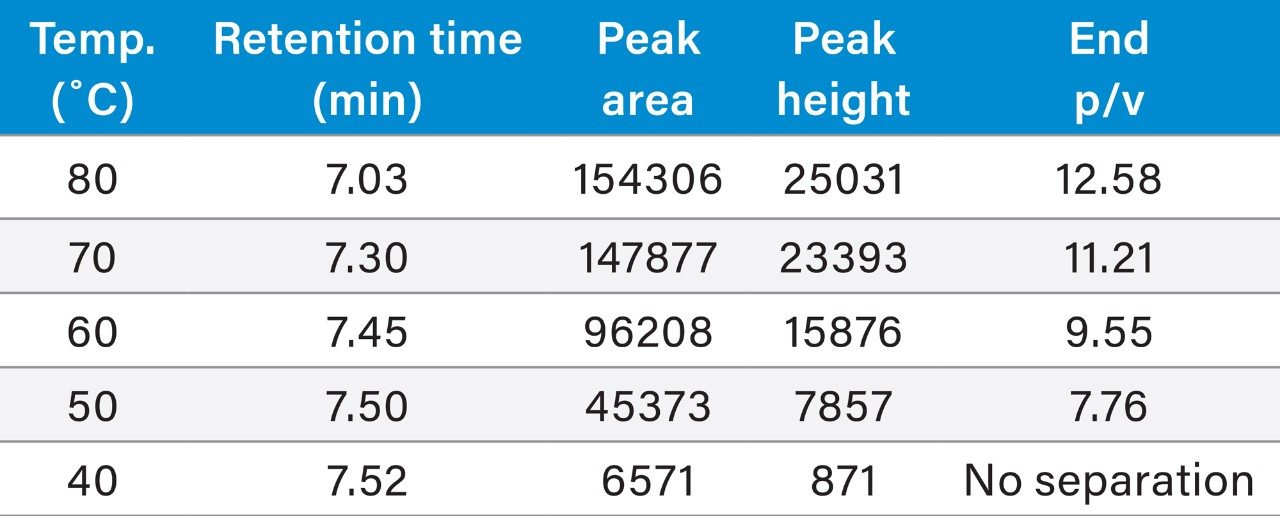
To improve protein resolution and recovery, the separation of an intact mAb is commonly conducted at a high temperature (≥80 °C) to assist desorption and improve the characterization and quality of data for quantitation. However, separation at a lower temperature is still desired to prevent potential thermal induced on-column degradation of samples. The BioResolve RP mAb Polyphenyl Column contains solid-core, silica particles with a novel polyphenyl bonded phase that reduces secondary interactions and facilitates desorption, which could potentially alleviate some of the temperature dependence for mAb separations. As a proof of concept, an antibody standard, Waters Intact mAb Mass Check Standard, was used to evaluate the performance of a BioResolve RP mAb Polyphenyl Column at various column separation temperatures. To establish a baseline for assessing recovery, a fast gradient method was developed for maximized peak-to-peak resolution at 80 °C. As shown in Figure 1 (red trace), using a 10 min gradient from 25–45% acetonitrile with 0.1% TFA, multiple impurities (i.e., shoulder peaks) were resolved from the main peak. Using this gradient method, a temperature study was carried out from 80 to 40 °C (Figure 1). Table 1 contains the metrics of peak integration at different temperatures, including retention time, peak area, peak height, and peak-to-valley ratio between the main peak and impurity 2. Results showed that the retention time of the eluted peak increased as the temperature decreased, with both resolution and peak area decreasing significantly at temperatures below 70 °C. At 40 °C, the eluted peak area was nearly zero while the baseline after the mAb elution was elevated significantly compared to the separation at 80 °C (Figure 1, inset), suggesting excessive adsorption of the antibody and a very unfavorable desorption rate. While not sufficiently robust for potential use in a validated method, these data suggested how acetonitrile based separations at lower temperature are feasible on the BioResolve RP mAb Polyphenyl Column versus other alternative column technologies, as previously demonstrated.5 Still, considering the above observations, it was warranted to pursue the development of new method conditions that could fully utilize the kinetic advantages and unique selectivity of the BioResolve RP mAb Polyphenyl Column and also increase protein recovery at lower temperatures.
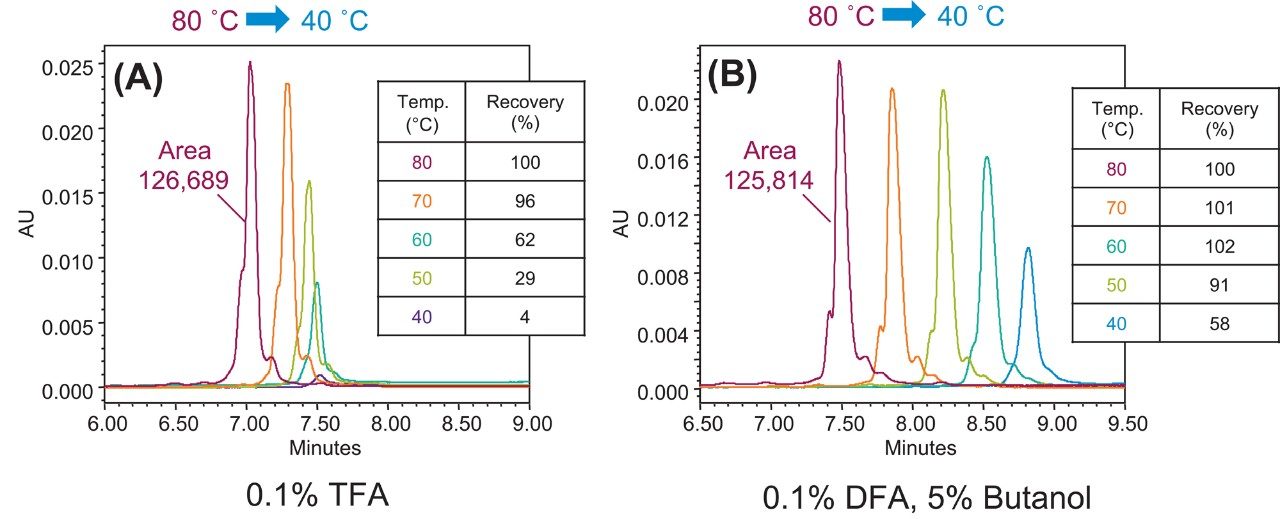
A common strategy to improve the recovery of proteins is to use different mobile phase additives, such as short chain alcohols or weaker ion-pairing reagents, to reduce adsorption as well as secondary interactions.3,6 In this case, a method was developed using a weaker ion-pairing reagent, difluoroacetic acid (DFA), in lieu of TFA. In addition, n-butanol was added as a co-solvent to the mobile-phase system. The TFA-based mAb separation was re-optimized using the new solvent additive and ion-pairing reagent to obtain similar retentivity as the chromatogram shown in Figure 1. To facilitate data comparison across conditions, the data were normalized by calculating recovery as the ratio of eluted peak area relative to the peak area observed at 80 °C. As shown in Figure 2, comparable recovery was obtained at 80 °C for the mAb peak using TFA (Figure 2A) or DFA with 5% n-butanol (Figure 2B). As postulated, the new mobile-phase conditions (Figure 2B) showed significantly higher recovery at temperatures below 70 °C as demonstrated by the calculated recovery at each temperature point (Figure 2, insets). An unexpected benefit of the new mobile-phase conditions was the apparent improvement in resolution of the impurity peaks from the main mAb form at temperatures as low as 60 °C (Figure 2B). The above data suggest the new method conditions improve column performance by facilitating the desorption of an intact mAb. Based on these observations, a natural extension of this work is to assess if the new method conditions can be applied to other samples with the same improvement on recovery.
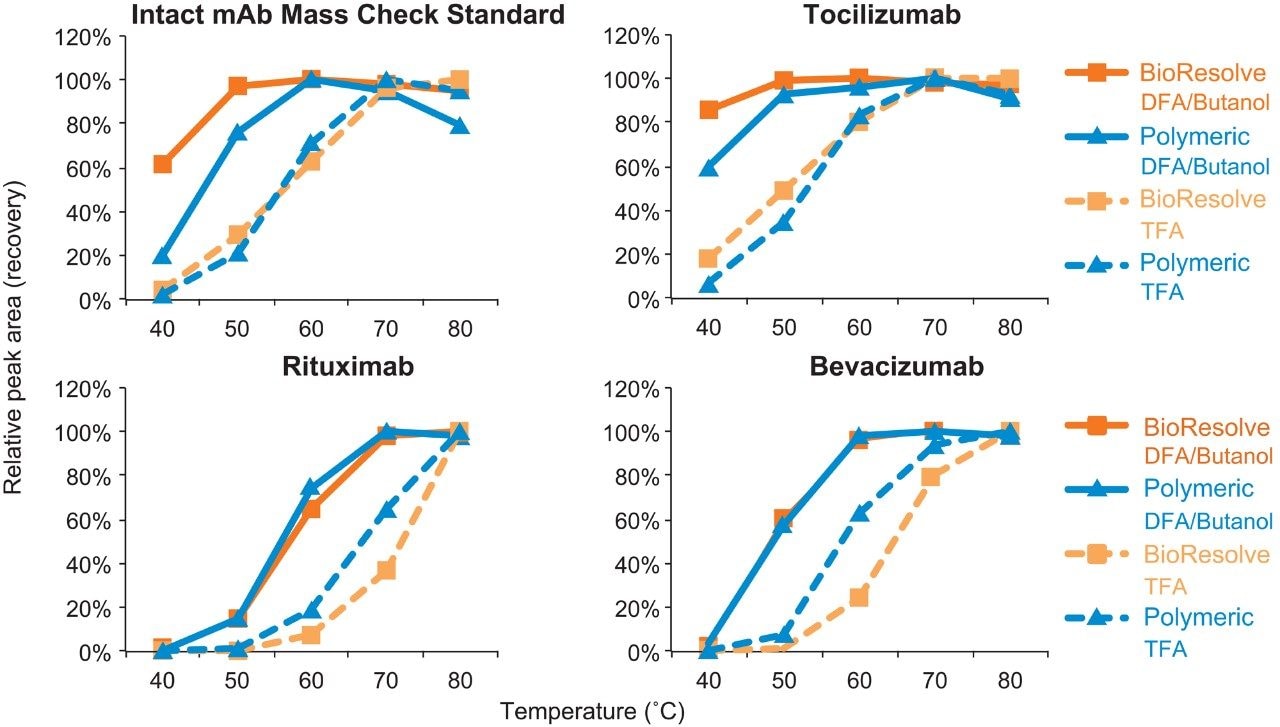
To investigate the applicability of this improved technique, analyses were performed on a panel of antibodies in addition to Waters Intact mAb Mass Check Standard, including Tocilizumab, Rituximab, and Bevacizumab. Due to the absence of silanol groups, a commercially available polymeric column was expected to provide the highest recovery for protein separations and it was therefore used as a comparison benchmark for the BioResolve RP mAb Polyphenyl Column. As shown in Figure 3, higher recovery was systematically observed in the separation of all antibodies using DFA/butanol (solid line data) versus TFA (dash line data). However, the improvement in recovery was observed to be sample dependent, which may be attributed to different adsorption rates caused by varying degrees of charge or hydrophobicity heterogeneity between mAb samples, or potentially even different melting temperatures among the various mAbs. Generally, the order of recovery relative to temperature was Waters Intact mAb Mass Check Standard ≈ Tocilizumab > Bevacizumab > Rituximab. Nevertheless, across the board, the BioResolve RP mAb Polyphenyl Column showed either higher or comparable protein recovery when compared to the polymeric column. Collectively, this study demonstrates that the BioResolve RP mAb Polyphenyl Column can be used with less aggressive separation conditions to develop efficient mAb separations that minimize sample degradation and lengthen column lifetime.
This work has demonstrated that lower temperature, high recovery separation methods for intact antibodies can be readily developed using a BioResolve RP mAb Polyphenyl Column. Using silica-based, solid-core particles, containing 450Å pores, and a novel polyphenyl bonded phase, the BioResolve RP mAb Polyphenyl Column was shown to increase the efficiency and selectivity of intact antibody separations while improving recovery at lower on-column temperatures. While recovery across varying temperatures was found to be sample dependent, it was seen that the BioResolve RP mAb Polyphenyl Column provided higher or comparable recovery compared to a leading commercially available polymeric column. In summary, the BioResolve RP mAb Polyphenyl Column is an ideal separation media for the analysis of mAbs while its performance characteristics can be readily used to improve the quality of existing and future RPLC-based assays.
720006220, February 2018