In this application note, we demonstrate the application of the Ostro Pass-through Sample Preparation Plate for plasma phospholipid removal prior to the LC-MS analysis of genfitinib.
Quantitative bioanalysis provides accurate and precise systemic drug concentration level data facilitating successful decision making in both drug discovery and drug development. This data is used in discovery for compound comparison and in development to perform species to species comparison as well as providing the pharmacokinetic data for dose ranging studies. LC-MS is the platform of choice for small molecule quantitative bioanalysis. Successful LC-MS bioanalysis relies upon robust, reproducible sample preparation to provide protein-free extracts allowing candidate pharmaceutical concentrations to be measured while maintaining instrument performance and minimizing downtime.
Protein precipitation is the simplest and least expensive form of plasma/serum sample preparation. It employs either organic solvent or aqueous acids to precipitate by reducing the solvation layer around the proteins causing them to aggregate and drop out of solution. However, this approach results in a complex solution which contains the remaining constituents in plasma, which are soluble in the aqueous organic solvent mixture, such as lipids, amines, and small organic acids. These remaining matrix components can foul the MS source, reducing MS response and result in system downtime. Solid-phase and liquid-liquid extraction result in a significantly cleaner extract, and afford the opportunity for a concentration step, giving higher sensitivity. However, both processes are time consuming and require method development.
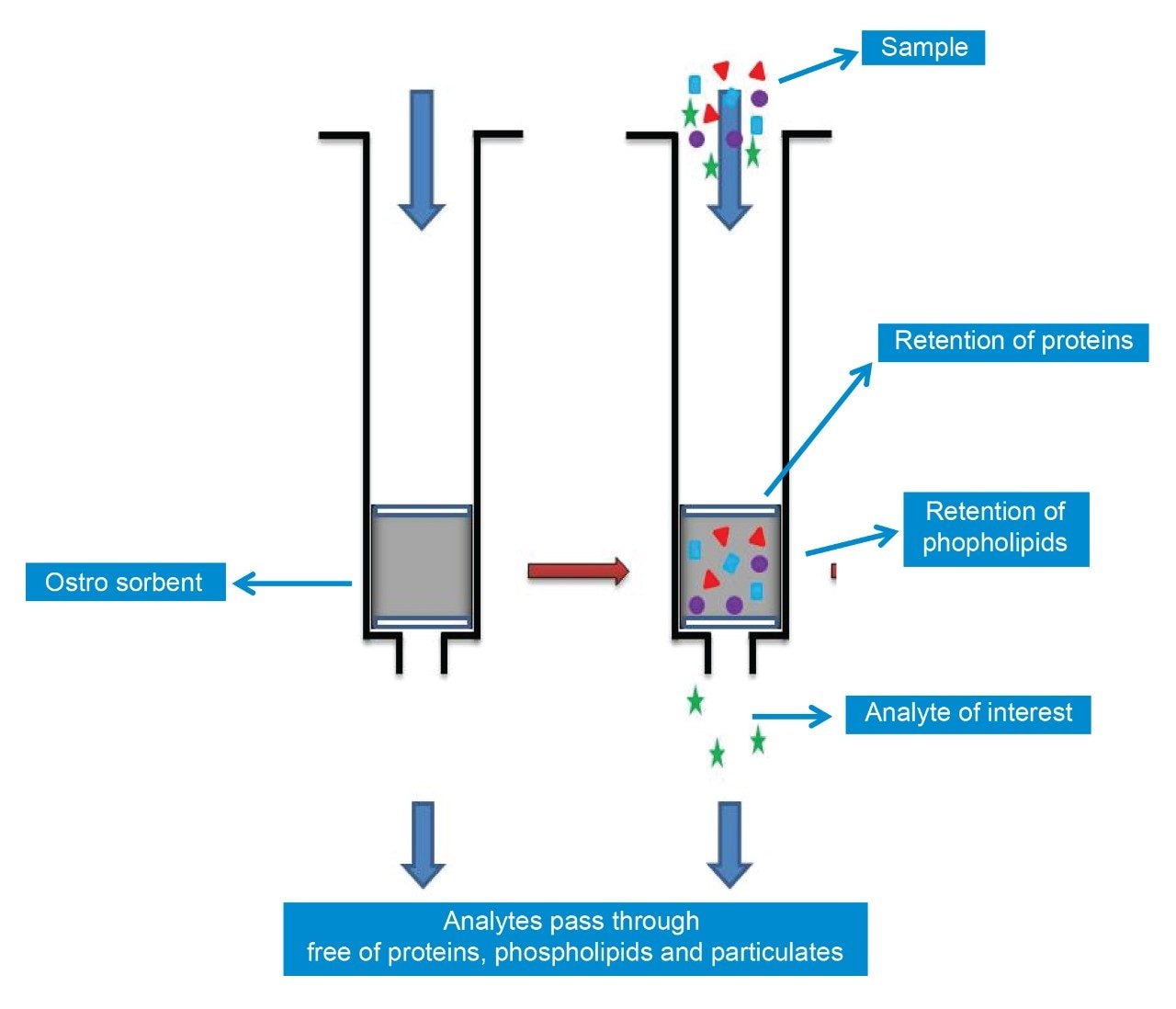
Ostro Pass-through Sample Preparation Plates provide a novel solution for phospholipid and protein removal. They facilitate rapid, simple sample preparation (for plasma/serum) without the need for complicated method development, while effecting sample clean up. In this application note, we demonstrate the application of the Ostro Pass-through Sample Preparation Plate for plasma phospholipid removal prior to the LC-MS analysis of genfitinib.
Mouse plasma samples were prepared by protein precipitation with four times the volume of methanol or methanol containing 1% formic acid.
For the standard method 10 μL of plasma was mixed with 40 μL of methanol containing the stable labelled isotope of genfitinib (Figure 1), vortex mixed and centrifuged at 25,000 g for five min. Ten microliters of the resulting supernatant was diluted 1 in 50 with 490 μL of 50:50 methanol:water.
For the Ostro Plate method, 10 μL of sample was added to the Ostro Plate, to which was added 40 μL of methanol containing the stable labelled isotope of genfitinib, a further 200 μL of methanol containing 1% formic acid was added to each sample and the sample mixed by aspiration (Figure 2). The sample was then drawn through the Ostro Plate under vacuum. 50 μL of the resulting solution was diluted 1 in 10 with 450 μL of 50:50 methanol:water
Example parameters are shown below, adjust as necessary for your conditions. If a non-Waters brand equipment or disposable is required for performing the procedure, it should be detailed.
|
System: |
ACQUITY UPLC I-Class PLUS |
|
Detection: |
Xevo TQ-S micro Tandem Quadrupole Mass Spectrometer |
|
Column: |
ACQUITY UPLC BEH C18, 1.7 μm, 2.1 × 100 mm (p/n: 186002352) |
|
Column temp.: |
60 °C |
|
Sample temp.: |
6 °C |
|
Injection volume: |
2 μL |
|
Flow rate: |
650 μL/min |
|
Mobile phase A: |
0.1% formic acid in water plus 10 mM ammonium acetate |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
Gradient: |
Linear gradient from 5–50% B over 2.9 min followed by 1.5 min flush with 95% B |
|
System: |
Xevo TQ-S micro |
|
Ionization mode: |
Positive ion ESI |
|
MS detection: |
Phospholipids precursors of 184 |
|
MRM detection: |
Iressa 446.6⇒ 128.23 |
|
MRM detection: |
Internal standard (d6) 452.6⇒134.23 |
|
Capillary voltage: |
2 kV |
|
Collision energy: |
33 |
|
Cone voltage: |
30 V |
|
Data acquisition software: |
MassLynx v4.2 |
|
Data processing and quantification software: |
TargetLynx |
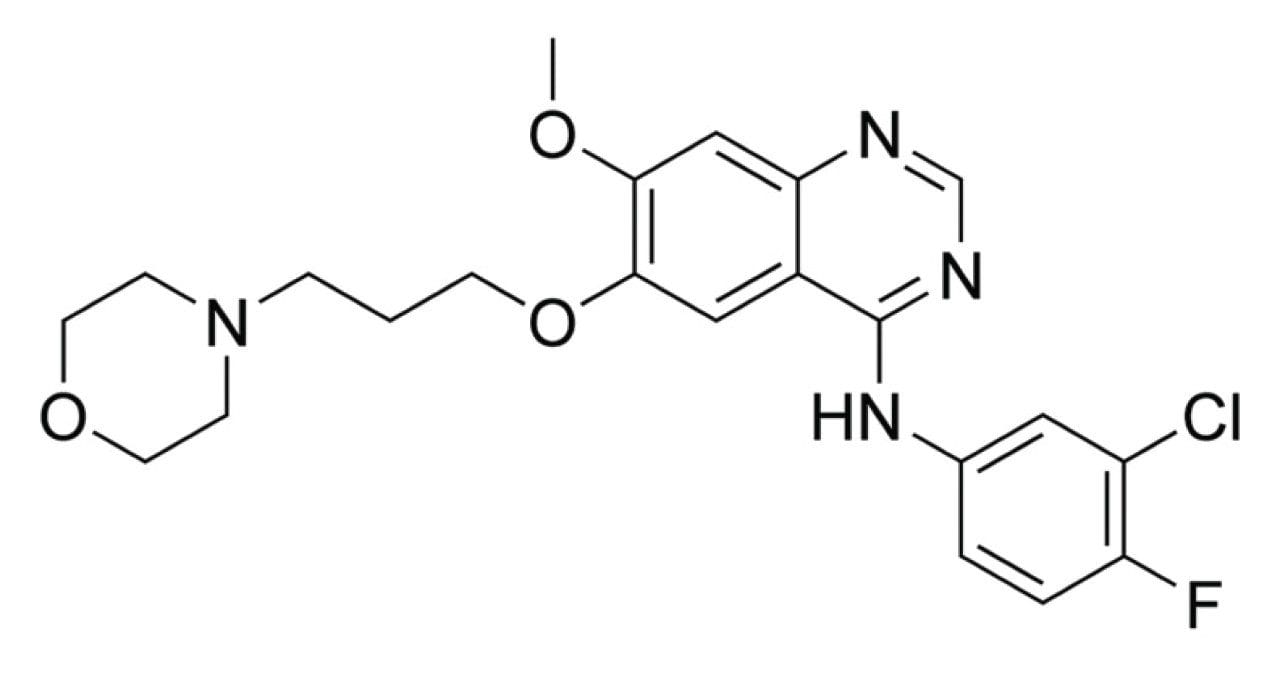
Phospholipids have been well documented to impair the performance of LC-MS quantitative assays due to ion suppression and source contamination.1,2 Removing these phospholipids from the matrix improves assay sensitivity, robustness, and precision. To evaluate the Ostro Plates for phospholipid removal, a routine discovery DMPK assay for the quantification of gefitinib was selected. Gefitinib is a medicine prescribed for the treatment of breast, lung, and other cancers and was marketed as Iressa (Figure 1). The molecular formula of gefitinib is C22H24ClFN4O3 giving an average molecular mass of 446.902 g/mol and a mono isotopic molecular mass of 446.152. The compound has a bioavailability of 60%, exhibits >90% protein binding, and undergoes first-pass metabolism in the liver (CYP3A4) to form the o-desmethyl metabolite.3
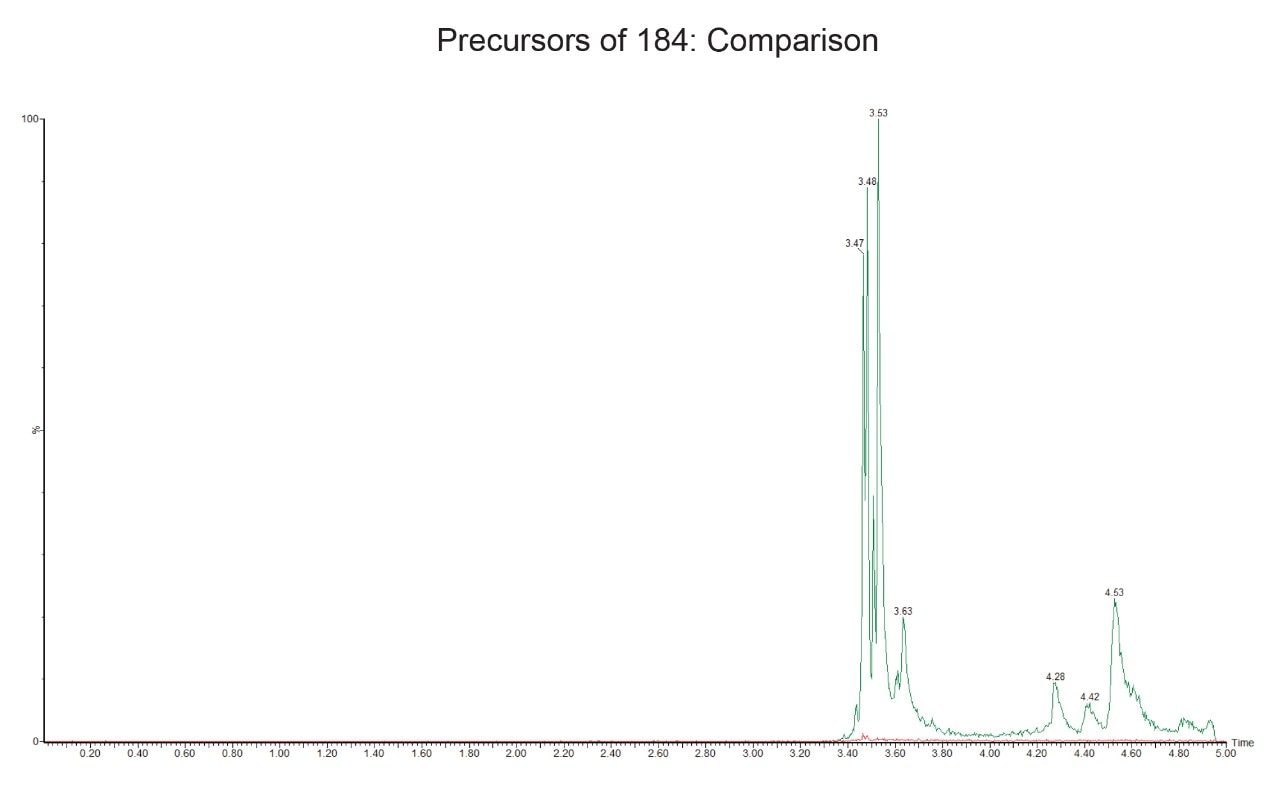
Previous data has shown that protein precipitation with acetonitrile yielded poor results, thus for this experiment methanol was employed for the protein precipitation. The same solution was also used for the Ostro Plate extraction of gefitinib. The samples were diluted post extraction to ensure that the final volumes and concentrations remained constant. The samples were analyzed using reversed-phase gradient chromatography and monitored using the precursors of m/z = 184 in positive ion mode employed to detect the phospholipid components in the sample. The resulting chromatograms obtained using the organic solvent protein precipitation and Ostro Pass-through Sample Preparation Plates are displayed in Figure 3; here we can see that the sample extracted with the Ostro Plates show significantly less signal for the phospholipids (red trace) than the sample extracted using the organic solvent method (green trace).
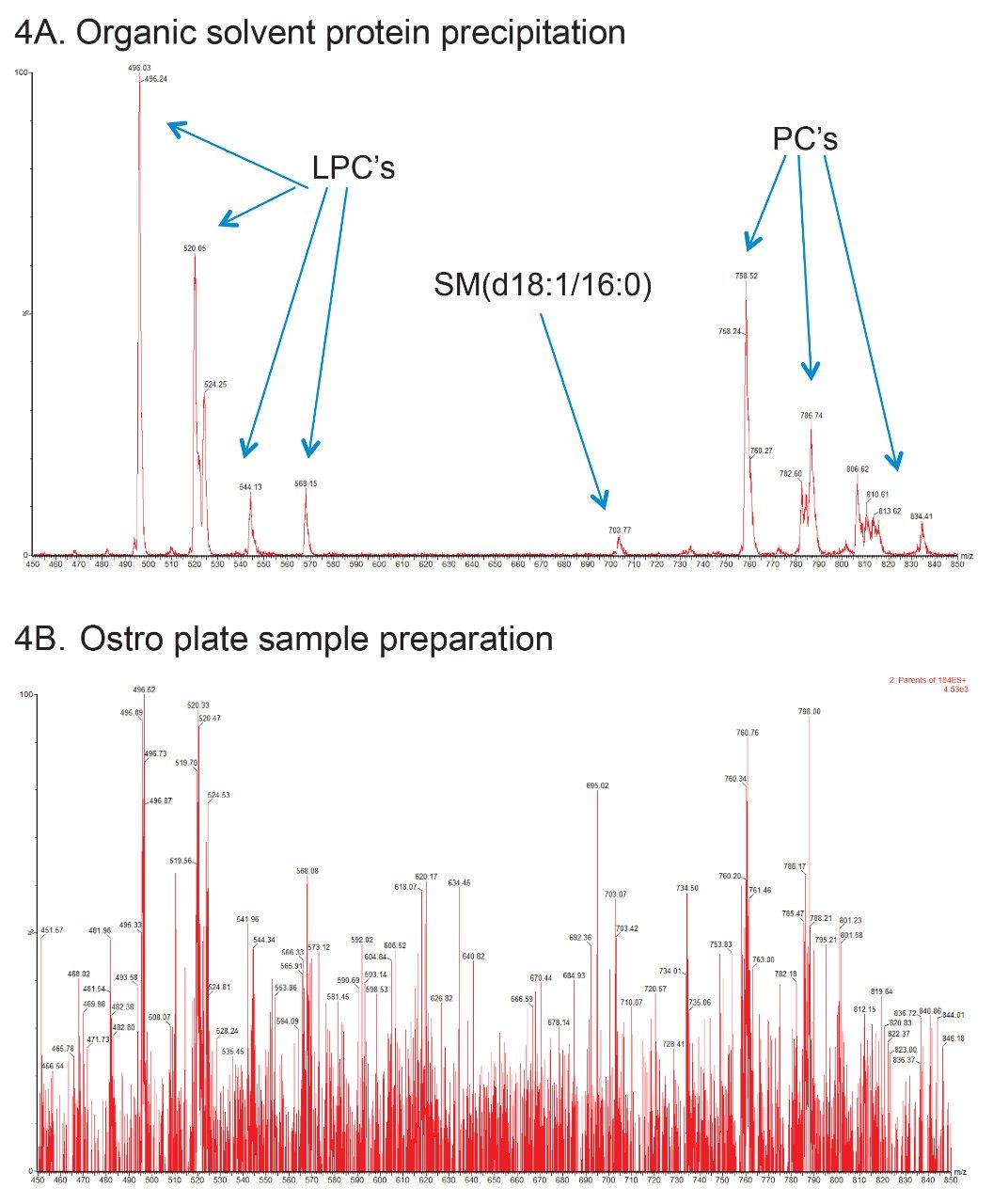
To further illustrate the phospholipid contamination in the sample, the spectra was combined over the chromatographic region 3.4–4.8 min. The spectra derived is displayed in Figure 4 organic (solvent method upper trace, Ostro Plate method lower trace). Here we can see that there is significant MS signal in the organic solvent method sample related to the lysophosphatidylcholines (LPC’s) precursors m/z = 496, 520, 524, 544, and 568, the phosphatidylcholines (PC’S) precursor m/z = 758, 782, 786, 806, and 834. There is also a signal clearly visible for the sphingomyelin lipid (d18:1/16:0). This result can be compared to the same experiment performed for the Ostro Plate extracted sample (lower trace) where the same region of the chromatogram showed no signal from the precursors of m/z = 184 transition. This result illustrates that the Ostro Plate effectively removes the phospholipids and proteins from the plasma sample.
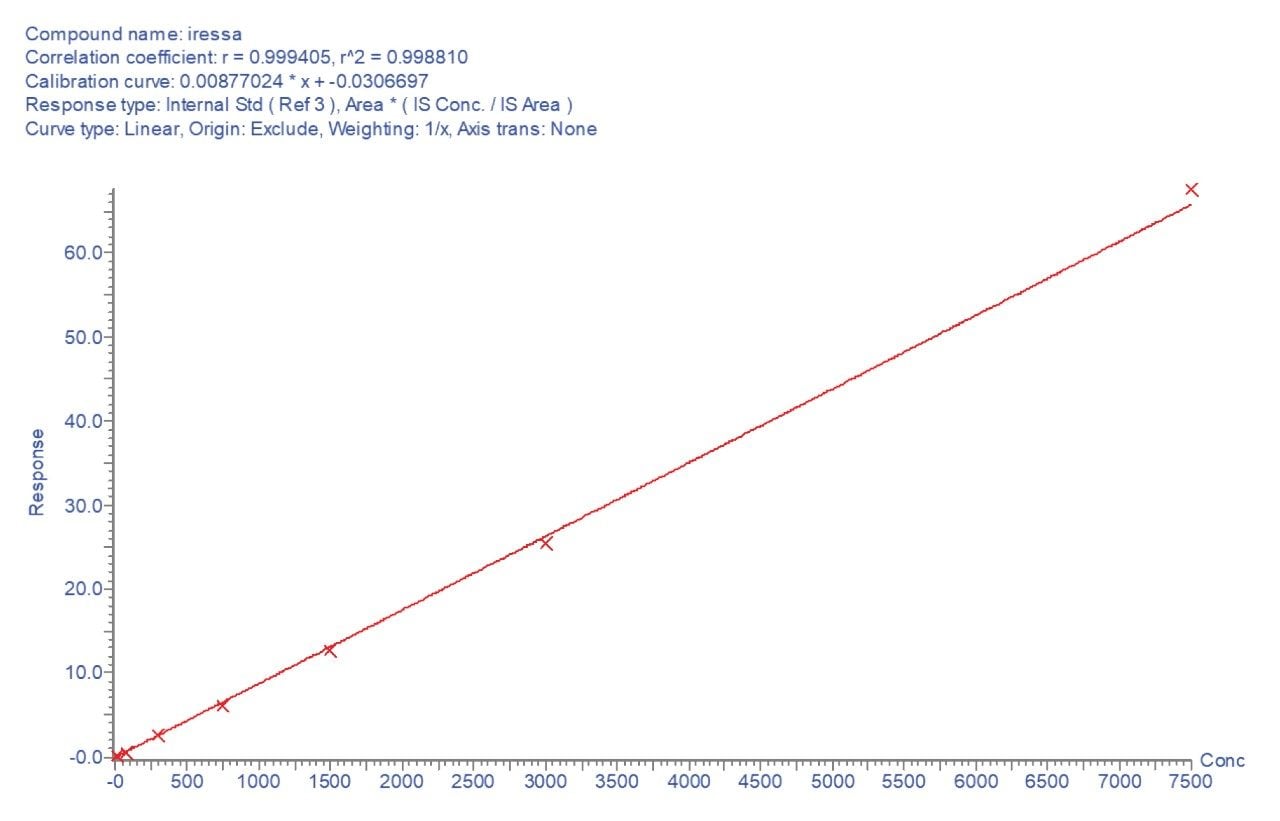
The typical systemic exposure levels for gefitinib range from 50–7000 ng/mL over a 24 hr period following dosing. The assay developed for gefitinib was demonstrated to be linear for gefitinib over the range of 15–7500 ng/mL (Figure 5) using 1/x weighting and internal standard calibration. The correlation coefficient was determined to be (r2) 0.998810 with an intercept of 0.03067. The signal response for the gefitinib standards and QCs were within 95% of that obtained by the protein precipitation methodology, thus demonstrating that no analyte absorption onto the plate occurred, even at low concentrations.
The removal of contaminants from a biological fluid matrix such as plasma, serum, or urine is key to reducing ion suppression and minimizing MS source contamination. Waters Ostro Pass-through Sample Preparation Plate removed all the phospholipid components from the plasma sample using a simple generic approach without the need for complicated sample preparation. There was no need for complicated method development and optimization. Comparison to the organic solvent protein precipitation method showed that there was no reduction in analyte response. The resulting assay was determined to have linear response over the range of 15–7500 ng/mL.
720006817, April 2020