
For research use only. Not for use in diagnostic procedures.
Vitamin K1 (phylloquinone) analysis using electrospray ionization mass spectrometry is challenging due to the hydrophobic nature of the molecule and lack of ionization sites. An additional issue is the low concentration of vitamin K1, which may be as low or even lower than 0.1 ng/mL in serum.
A new clinical research method for the analysis of vitamin K1 in serum has been developed using UPLC-MS/MS with electrospray ionization. Sample preparation uses PRiME µElution solid phase extraction with 200 µL of serum. Using an ACQUITY UPLC I-Class FTN with ACQUITY UPLC HSS PFP Column chemistry and a water/methanol/ammonium fluoride gradient with a Xevo TQ-S micro, a lower limit of the measuring interval of 0.05 ng/mL was achieved, with reproducible precision and compensation for ion suppression by the stable labeled internal standard, 13C6-vitamin K1.
Vitamin K refers to a group of fat-soluble vitamins absorbed in the intestine. It consists of vitamin K1 and several forms of the menaquinone series vitamin K2.
Vitamin K is a lipophilic vitamin and is present in serum at low concentrations, therefore laboratories traditionally use techniques such as supercritical fluid chromatography (SLC) or atmospheric pressure chemical ionization (APCI) to improve the analytical sensitivity and selectivity of the results.
In this application note a UPLC-MS/MS method using electrospray ionization (ESI) has been developed demonstrating high analytical sensitivity along with a fast run time for use in clinical research. In this application note a clinical research method for the extraction and analysis of vitamin K1 using UPLC-MS/MS electrospray ionization with only a 3-minute run time is described. Chromatography of extracted samples was performed on an ACQUITY UPLC I-Class System using an ACQUITY UPLC HSS PFP Column followed by mass detection on a Xevo TQ-S micro Tandem Quadrupole Mass Spectrometer (Figure 1).

600 µL of ethanol containing internal standard was added to 200 µL serum. Samples were mixed and centrifuged. Supernatant was loaded onto an Oasis PRiME HLB µElution Plate, washed, then eluted using heptane. Following drying, extracts were reconstituted in methanol and water.
|
LC system: |
ACQUITY UPLC I-Class FTN |
|
Detection: |
Xevo TQ-S micro |
|
Vials: |
96-well Plate with Extended 1 mL Glass Inserts (Waters P/N 186000855) |
|
Column(s): |
ACQUITY UPLC HSS PFP 2.1 x 50 mm, 1.8 µm (Waters P/N 186005965) |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
20 µL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
Water with 0.05 mM ammonium fluoride |
|
Mobile phase B: |
Methanol with 0.05 mM ammonium fluoride |
|
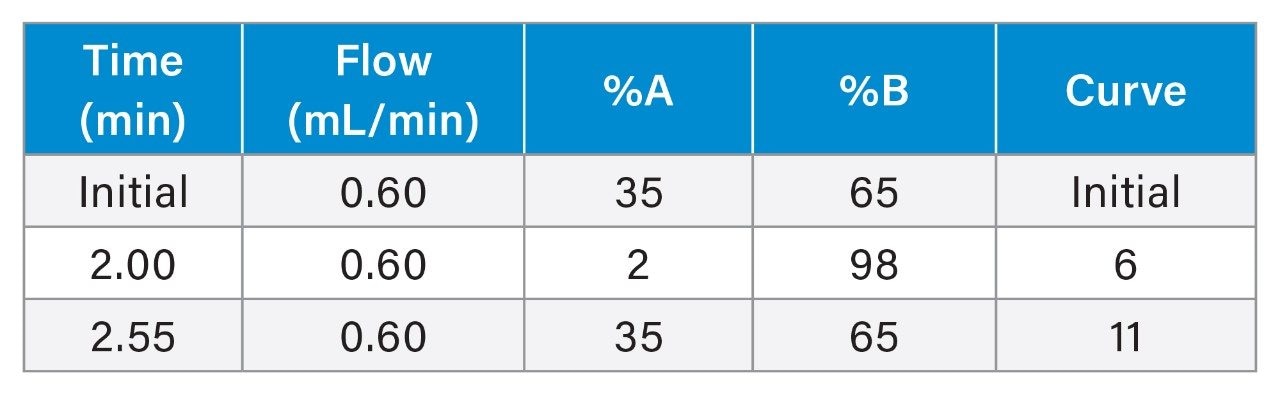
Gradient: |
See table |
|
MS system: |
Xevo TQ-S micro |
|
Ionization mode: |
Electrospray ionization in positive mode |
|
Acquisition mode: |
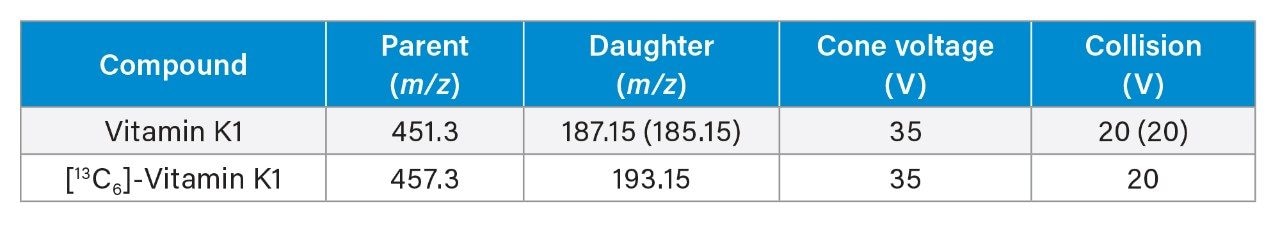
MRM (Multiple Reaction Monitoring) See table for details |
|
Capillary voltage: |
1.2 kV |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
650 °C |
|
MS software: |
MassLynx v4.2 with TargetLynx XS Application Manager |
Below is an example of a chromatogram of vitamin K1 and its internal standard from a human serum sample whose concentration is 0.14 ng/mL (Figure 2).
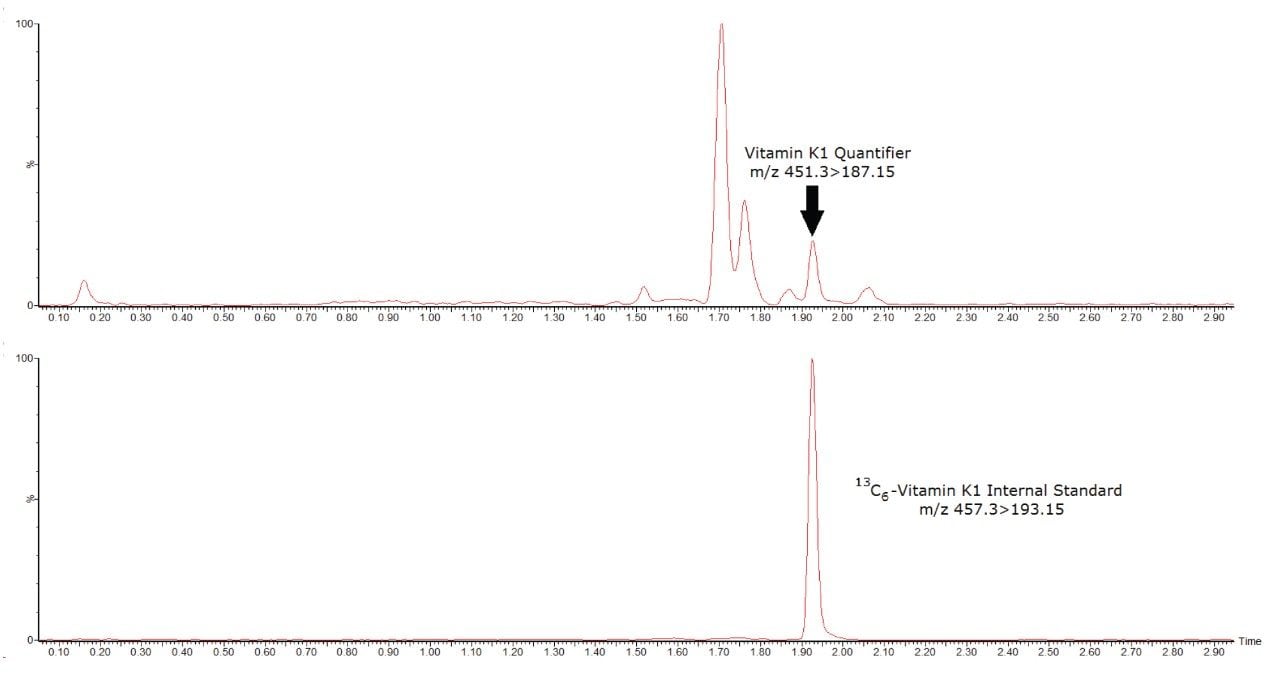
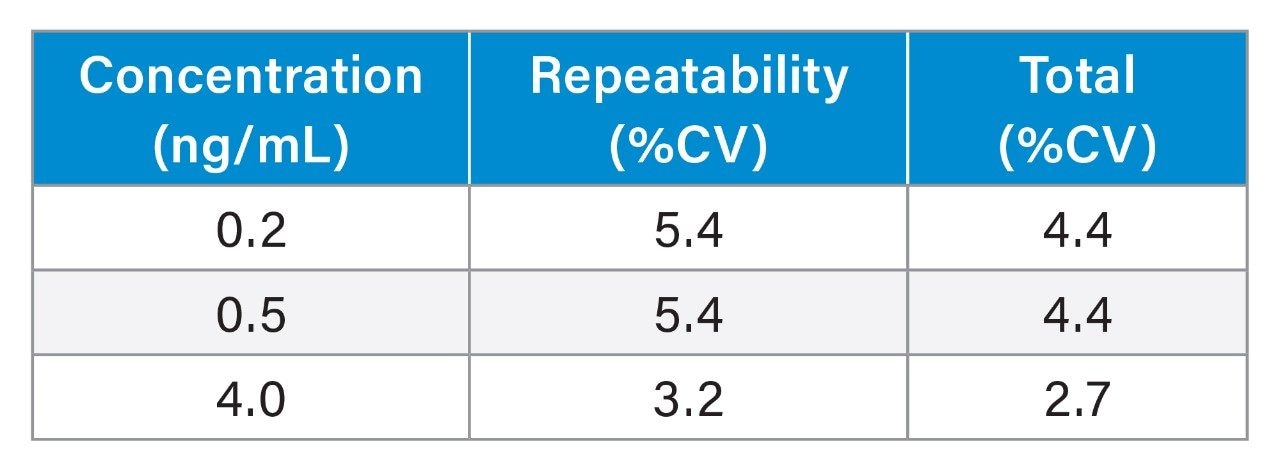
No system carryover was observed from high concentration samples into subsequent blank injections. Precision was assessed by extracting and measuring 5 replicates of samples across 5 days (n=25). Total precision and repeatability were ≤5.4% CV at the QC concentrations of 0.2, 0.5, and 4 ng/mL for vitamin K1 (Table 1)
Analytical sensitivity was assessed by extracting and quantifying 10 replicates of low concentration vitamin K1 samples prepared in stripped serum over 5 days. The lower limit of the measuring interval (LLMI) was determined to be the lowest concentration at which precision was ≤20% CV and bias ≤15% CV. Additional consideration was made of the concentration at which the system could consistently measure the difference between the blank serum pool, with its endogenous concentration, and the pool being evaluated. The LLMI was considered to be at a concentration of 0.05 ng/mL (Table 2).
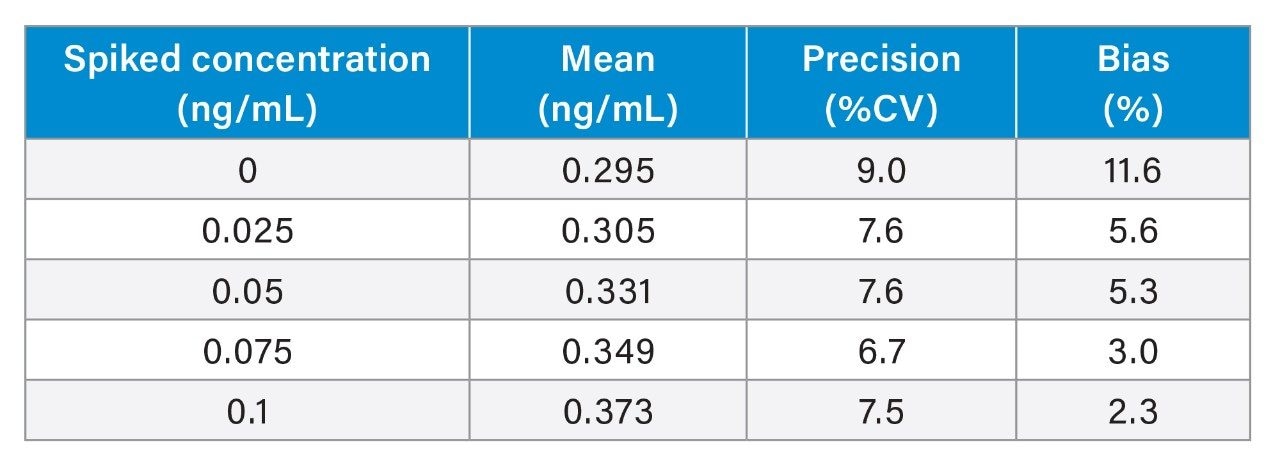
The method was shown to be linear across the range of 0.077–26 ng/mL for vitamin K1, when low and high pools were mixed in known ratios over the range. All calibration lines in spiked stripped serum were linear with a coefficient of determination (r2) ≥0.995.
Typical endogenous interferences (albumin, bilirubin, creatinine, cholesterol, triglycerides, and uric acid) and exogenous compounds (retinol, alpha-tocopherol, vitamin K1 2,3-epoxide, vitamin K2 2,3-epoxide, and vitamin K2) were tested and recoveries of the test samples compared to controls were all within ±7.6%. A substance was deemed to interfere if a recovery range of 85–115% was exceeded.
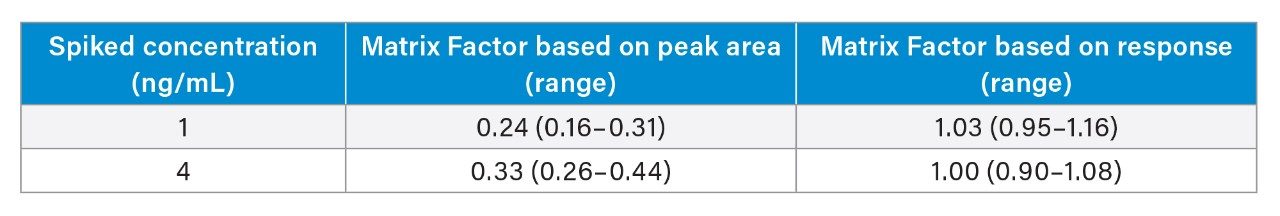
Matrix effect investigations were performed using donor serum samples from six individuals. The endogenous peak areas were separately quantified and post-spiked samples at low and high concentration levels were adjusted using the mean peak areas to enable comparison to solvent spiked samples (Table 3). Although significant suppression of vitamin K1 was observed, the internal standard was demonstrated to adequately compensate.
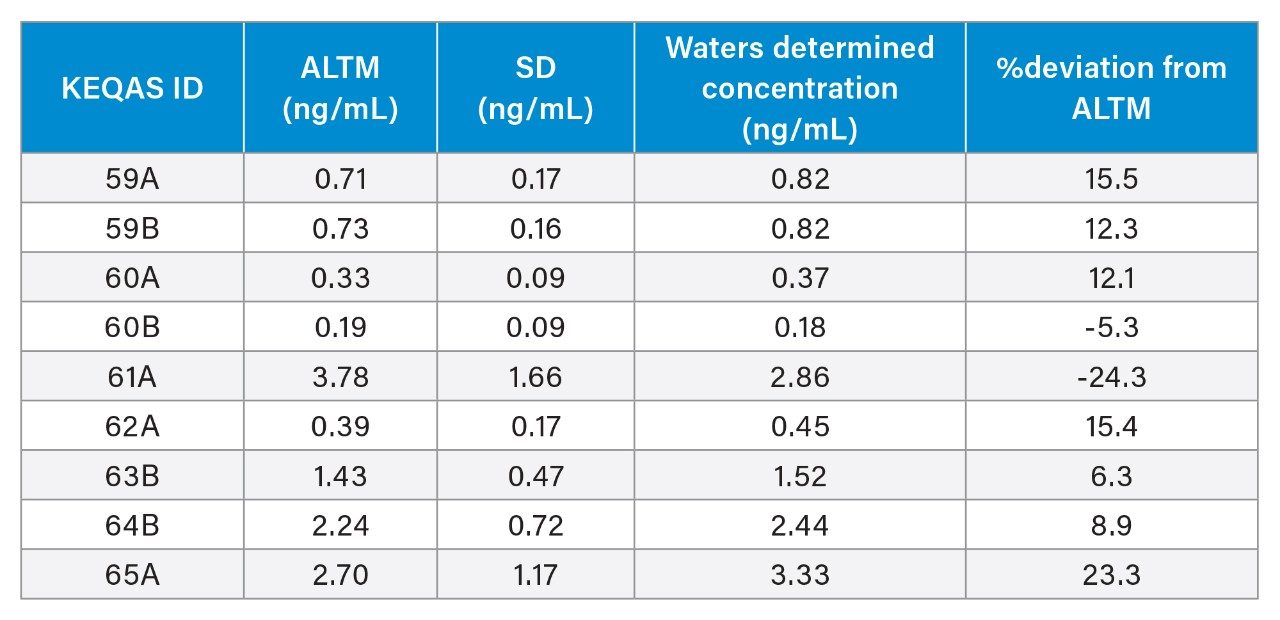
Accuracy was assessed by analyzing 9 KEQAS (Vitamin K External Quality Assurance Scheme, London, UK) vitamin K1 samples with calculated concentrations compared to the ALTM (All Laboratory Trimmed Mean). The results can be seen in Table 4, showing good agreement with the scheme, with a mean bias of 7.1% over the range 0.19–3.78 ng/mL.
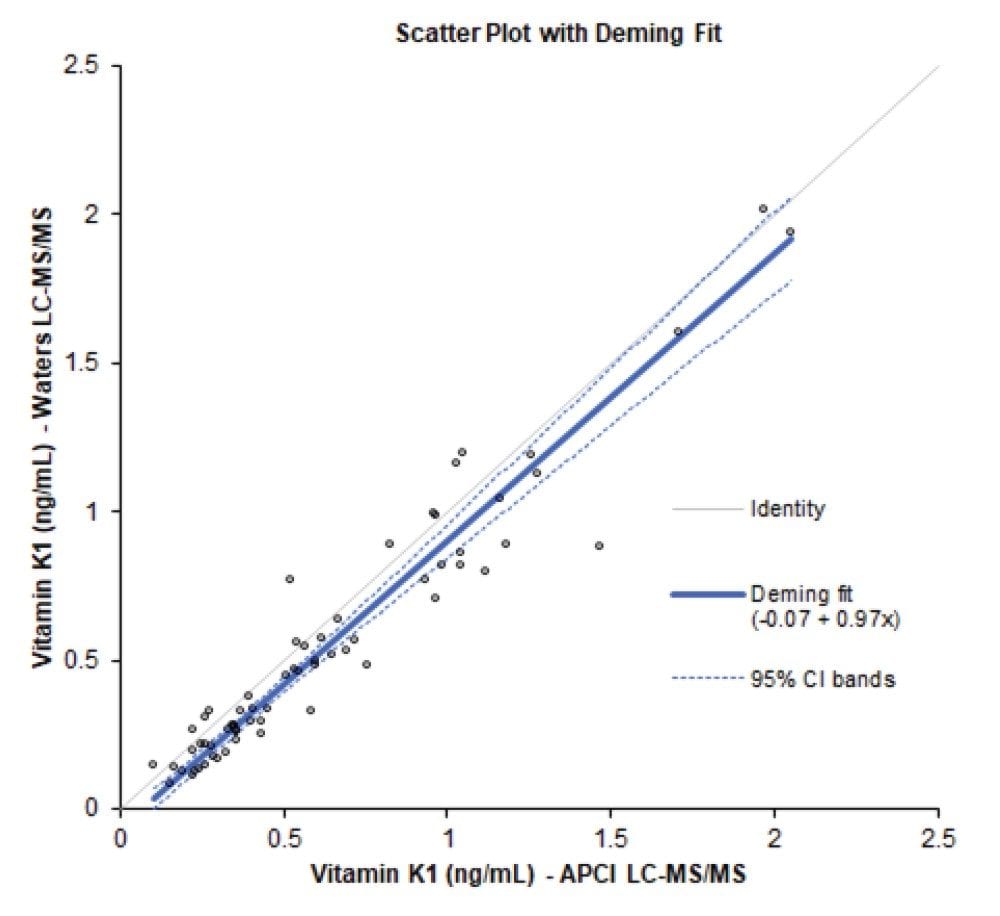
A set of anonymized serum samples were obtained (n=66, 0.11–2.05 ng/mL) for method comparison against an independently developed SPE-LC-MS/MS method using APCI. A Deming equation of y = 0.97x - 0.07 was obtained, showing statistically significant constant bias, though no significant proportional bias. The plot is presented in Figure 3.
Recoveries (extraction efficiency) over the QC concentrations ranged from 80.2–93.2%, with an overall mean of 84.9% and overall 8.6%CV.
A fast method has been developed for the challenging analysis of vitamin K1 in serum for clinical research. With a short run time, the method allows high throughput of samples. Analytical sensitivity investigations suggest vitamin K1 concentrations of ≤0.1 ng/mL in serum may be reliably detected. The method describes very good precision, absence of carryover, linearity across the required range, no interference from endogenous and exogenous compounds tested, absence of matrix effects due to the chosen internal standard, and consistent extraction efficiency. In addition, good agreement was observed for the method comparison to an independent LC-MS/MS method and agreement with EQA samples was also demonstrated.
Anne Schmedes at the Biochemistry and Immunology Laboratory Center (Vejle, Denmark) is thanked for the provision of samples for method comparison.
720007169, February 2021