Isocratic Separation of RNA Nucleotide Triphosphates Including Pseudouridine using an Atlantis™ Premier BEH™ Z-HILIC Column
Abstract
Synthetic RNA has gained popularity in recent years mainly as an avenue to therapeutic medications including vaccines for viruses such as SARS-CoV-2. The rapid growth of this technology has led to an increase in the number of facilities involved with creating and testing synthetic RNA. Prior to synthesis, the quality of the individual nucleotides must be assessed to ensure the highest quality product is being made. Furthermore, nucleotides can readily break down from their triphosphate to di- and monophosphate forms, potentially affecting the efficiency of the synthetic process. Having analytical methods to detect the breakdown products of these critical starting compounds is required. Previously, the analysis of nucleotide triphosphates was performed using mixed-mode columns or ion-pairing reversed phase chromatography. However, Hydrophilic Interaction Chromatography (HILIC) provides several advantages for nucleotide separations, including higher MS sensitivity. In the work described here, a method was developed for separating the four main RNA nucleotide triphosphates using an Atlantis Premier BEH Z-HILIC Column. Additionally, the modified nucleotide pseudouridine triphosphate was also analyzed with good separation from the isobaric uridine triphosphate in under fifteen minutes.
Benefits
- Baseline separation of the four RNA nucleotides along with a commonly used modification of uridine triphosphate was achieved
- Fifteen–minute isocratic separation of the nucleotides enables monitoring of nucleotide degradation
- MaxPeak™ High Performance Surfaces Technology produces good peak shapes for the nucleotides
Introduction
Synthetic RNA, especially messenger RNA (mRNA) is a relatively new technique for introducing proteins into a biological system. In the case of the Pfizer and Moderna SARS-CoV-2 vaccines, mRNA is used to create the spike protein, priming the immune system to recognize the virus, thus building up the immunological response in the event of actual infection.1 To express a specific protein, the correct mRNA sequence must first be created. Alterations to the mRNA could lead to a lack of protein expression, thereby making it ineffective. The science behind mRNA synthesis and subsequent protein expression is highly complex. One thing remains throughout this complexity, however: the need for high quality mRNA products that perform as expected when used by the general population.
In the creation of mRNA, the individual nucleotides must be arranged in a certain sequence. However, if those nucleotides are impure or are in the wrong form, i.e. a monophosphate instead of a triphosphate, then the creation of the mRNA could be affected. That is why using the highest purity nucleotides in synthetic processes is critical. One way to ensure that the nucleotides are of the highest purity is to test them using LC-UV or LC-MS to determine if any degradants or other impurities are present prior to introduction into synthetic processes. Reversed-phase LC, both with and without ion-pairing reagents, has been used to retain and characterize nucleotides.2–4 While the separations achieved using these techniques are reasonable, improvements can be made by using HILIC. HILIC is specifically designed for the retention and separation of polar analytes without ion pairing agents which are problematic for liquid chromatography-mass spectrometry (LC-MS) assays. Additionally, due to the high levels of organic solvent used in the mobile phase for HILIC, MS ionization is more effective, leading to high MS signals. Nucleotide separations can be achieved using HILIC as demonstrated in previous articles.5 The work cited documents a method to separate all twelve nucleotides, but requires a long run time, fifty minutes, and a complicated mobile phase. Performing a separation for incoming materials to be used in synthetic processes should be done as quickly as possible and would likely only be performed on one nucleotide or family of nucleotides at a time.
The work shown here describes the separation of the RNA nucleotide triphosphates, with the intent of creating a fast method to be used for quality control of incoming materials. A focus on separating the nucleotides was prioritized, especially for the isobaric compounds pseudouridine triphosphate and uridine triphosphate.
Experimental
Sample Description
Stock solutions of the nucleotides were created at a concentration of 1 mg/mL using the following sample diluents. Adenosine triphosphate required 30% water in acetonitrile. Cytidine triphosphate, uridine triphosphate, and guanosine triphosphate solutions were created using 25% water in acetonitrile. A sample mixture containing 50 µg/mL of each nucleotide was created using 90:10 acetonitrile:water as the diluent. Pseudouridine triphosphate stock solution (100 mM aq) diluted 1:100 with 93:7 acetonitrile:water prior to analysis and injected individually to avoid potential co-elutions.
LC Conditions
|
LC systems: |
ACQUITY™ UPLC™ H-Class PLUS with Quaternary Solvent Manager (QSM), Sample Manager Flow through needle (SM-FTN), Column Manager and Column manager Aux and PDA detector |
|
Detection: |
UV @ 260 nm |
|
Column: |
Atlantis Premier BEH Z-HILIC 2.1 x 100 mm, 2.5 µm (p/n: 186009986) |
|
Column temp.: |
20 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
1.0 µL |
|
Flow rate: |
0.2 mL/min (unless noted otherwise) |
|
Isocratic conditions: |
30:70 v/v Aqueous:Acetonitrile with 20 mM ammonium acetate pH 9.0 (unless noted otherwise) |
Data Management
|
Chromatography software: |
Empower™ 3 Feature Release 4 |
Results and Discussion
Based on a previously published separation, an Atlantis Premier BEH Z-HILIC Column was selected.5 A different configuration was used to improve the cycle time without sacrificing separation performance while also making the separation appropriate for typical UHPLC and HPLC instruments that are used in quality control. Additionally, a slightly higher buffer concentration (20 mM) was used to improve the peak shapes. An ACQUITY UPLC H-Class PLUS system with PDA was used to obtain UV assay results. The first conditions tested were those published using the 2.1 x 100 mm 2.5 µm column (Figure 1).
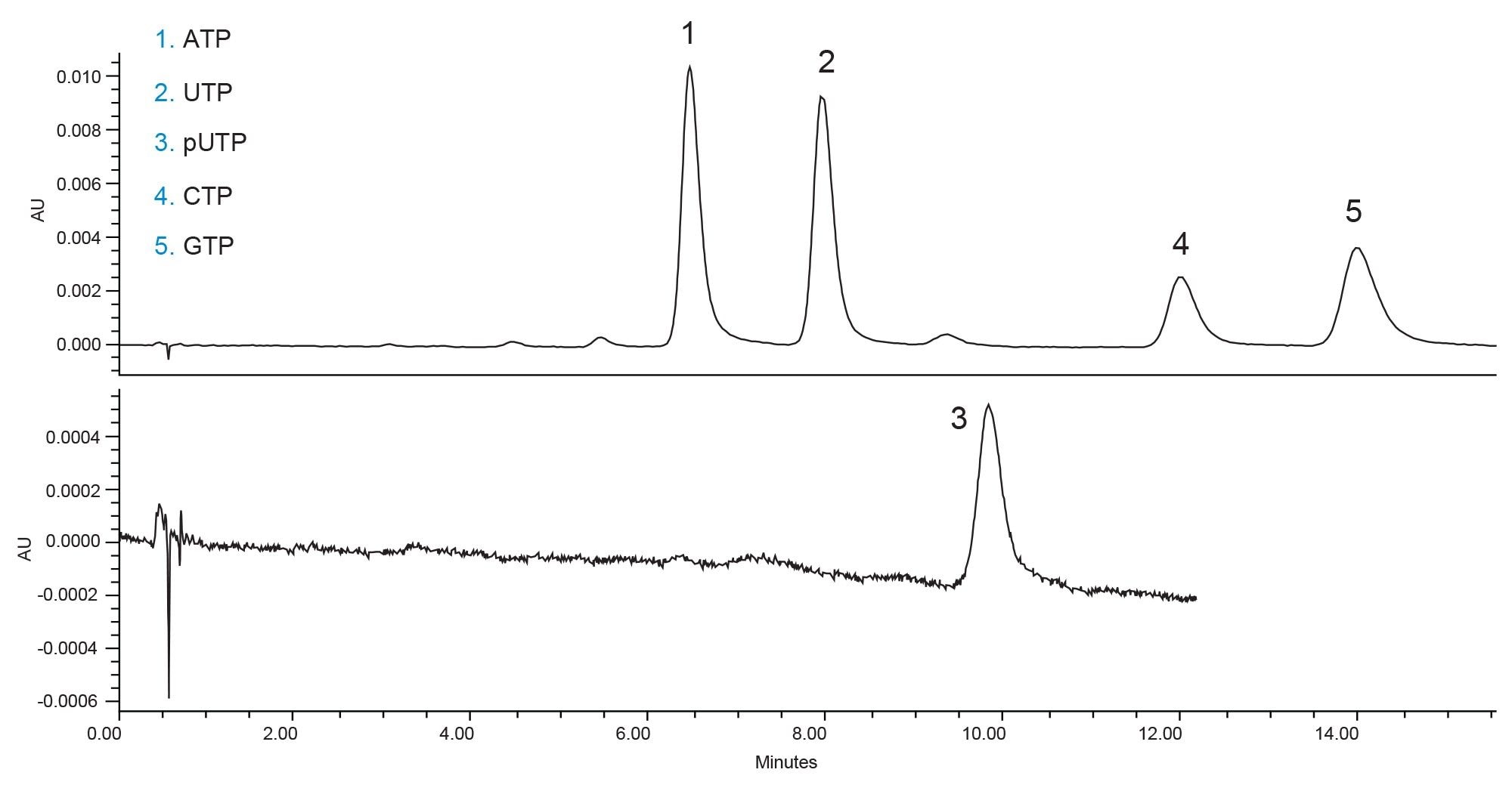
The documented method conditions can separate the five compounds of interest, however pseudouridine triphosphate (pUTP) has a very poor UV signal under these conditions. Additionally, both cytidine triphosphate (CTP) and guanosine triphosphate (GTP) show slight tailing and wide peaks. It should be noted that the small impurity peaks which are not labelled are the di- or monophosphate versions of the nucleotide triphosphates. Without any further optimization, the method would be appropriate for quality control testing of incoming nucleotides. However, the separation can be improved both for the peak shapes and the cycle time. Figure 2 shows the separation of the compounds without the methanol co-solvent in the mobile phase. Removing the methanol simplifies the mobile phase preparation and improves the peak shapes.
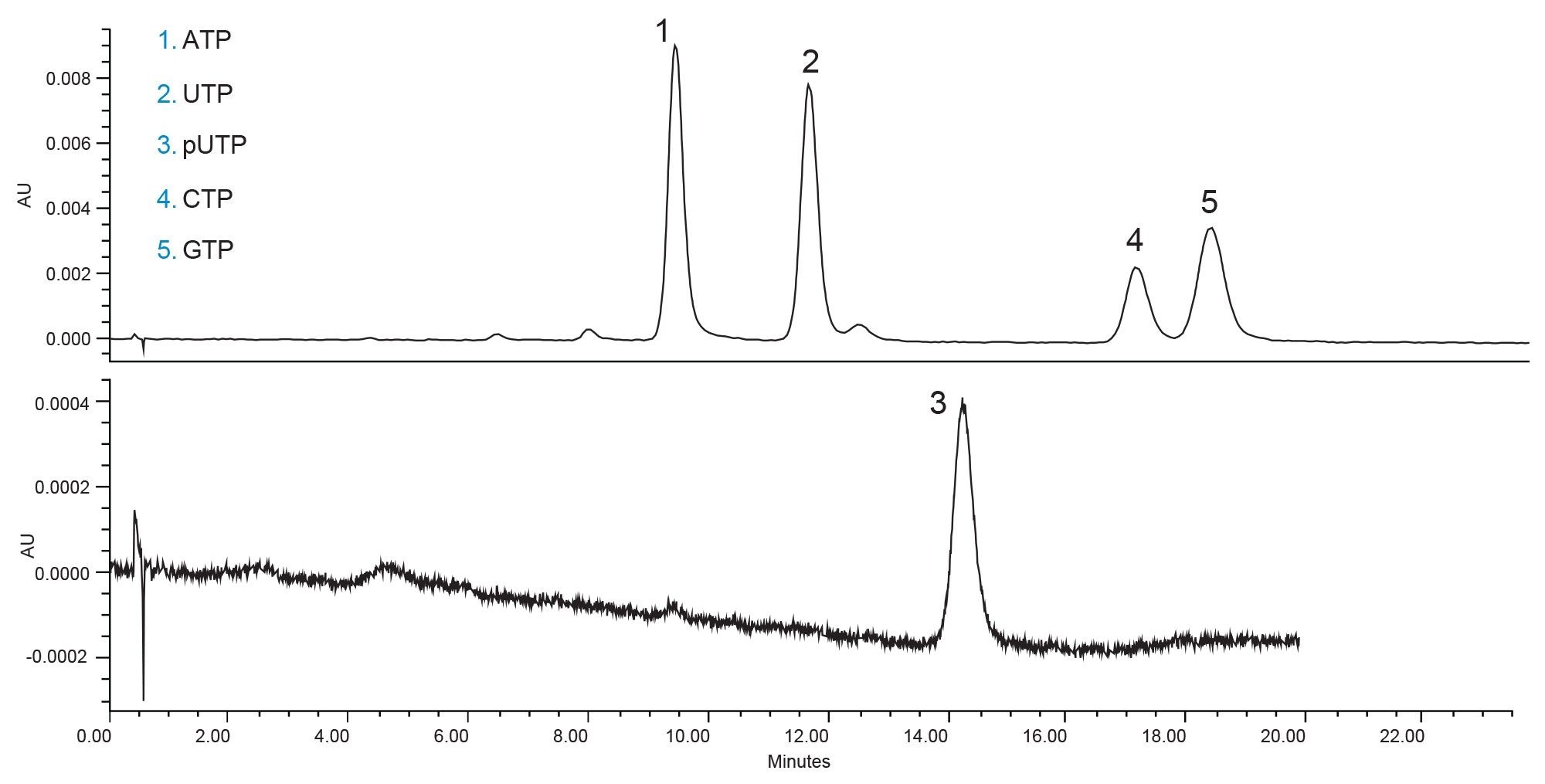
By removing the methanol and maintaining the same concentration of water in the mobile phase (25%) the mobile phase is weaker than when the methanol is present. This leads to longer elution times, in this case almost a 50% increase in retention. This is not ideal, but the peak shapes for CTP and GTP are improved. The mpUTP and UTP peaks are still very closely eluting but could be easily distinguished via MS if needed. A reduction of the cycle time would be preferred and therefore the method was next optimized by increasing the aqueous content of the mobile phase (Figure 3).
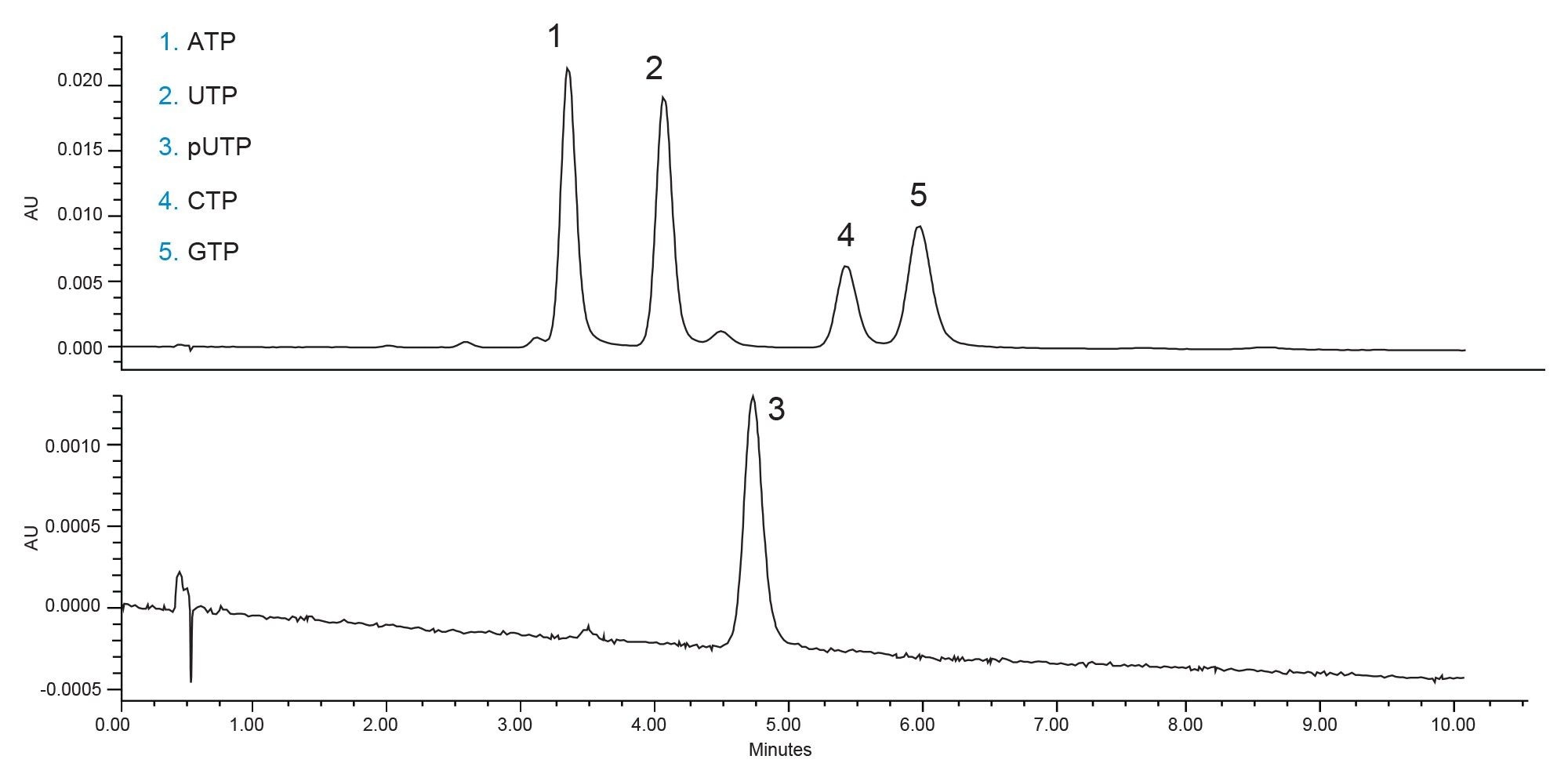
The increased aqueous concentration of the mobile phase reduced the retention times while maintaining good peak shapes. Some of the impurities co-elute with the nucleotides, most notably the peak eluting immediately before adenosine triphosphate (ATP), which is a diphosphate impurity. If the nucleotides were analyzed separately the impurities would be well separated from their respective triphosphate precursors. The separation under these conditions is very good but the slight co-elution between some impurity peaks and the main trisphosphate peaks is not ideal. One final optimization step was taken, to reduce the flow rate of the separation. This increases the column efficiency, improving the overall separation at the cost of increased cycle time. Figure 4 shows the separation of the nucleotides at a flow rate of 0.2 mL/min.
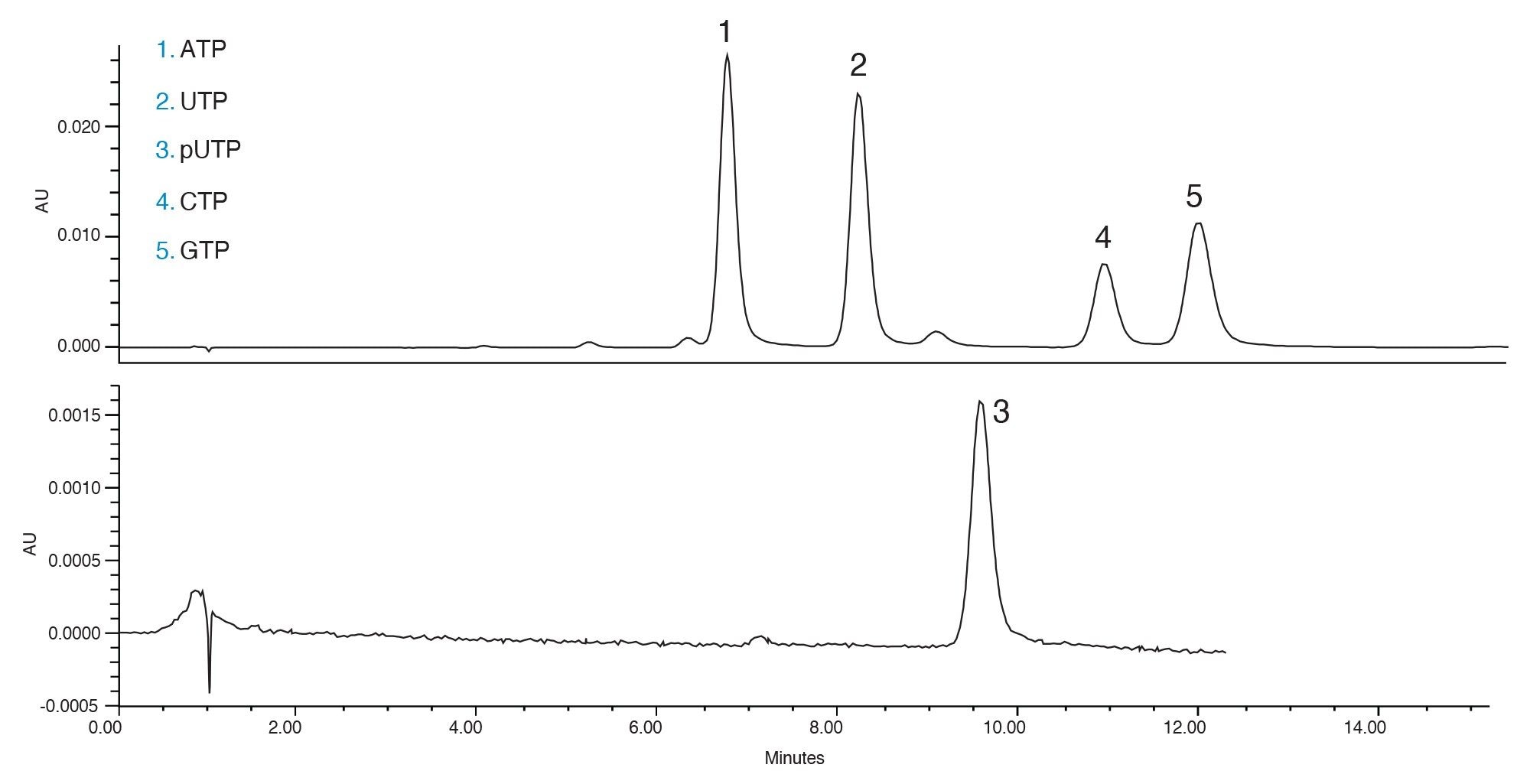
With the reduced flow rate, the separation of mpUTP and UTP is better. Additionally, more separation is achieved for ATP and the degradants that elute just before it. Using these conditions, the five main nucleotides of interest are separated. These conditions can be also used to analyze incoming samples of the nucleotides to determine their purity prior to use in the synthesis of mRNA.
Conclusion
A step-by-step approach was used to develop a HILIC method to separate RNA nucleotide triphosphates with baseline resolution for the four main RNA triphosphates as well as the commonly used pseudouridine triphosphate. This method could be used in a variety of ways including for the quality control of nucleotide triphosphates for use in RNA synthesis.
The HILIC separation was achieved using a simple mobile phase composed of 20 mM ammonium acetate at pH 9.0 in a 70:30 v/v acetonitrile:aqueous solution. This composition is appropriate for MS and UV detection. Good peak shapes were achieved for the compounds by utilizing MaxPeak Premier High Performance Surfaces (HPS) Technology. This technology mitigates ionic interactions between acidic moieties, like phosphate groups, and the metal surfaces present in an LC system and column.5 By using the Atlantis Premier BEH Z-HILIC Column which employs this technology a good result can be achieved for all five nucleotides in a single run.
References
- Xu S, Yang K, Li R, Zhang L. mRNA Vaccine Era- Mechanisms, Drug Platform and Clinical Prospection. International Journal of Molecular Sciences. (2021) 6582.
- Werner A. Reversed-phase and Ion-Pair Separations of Nucleotides, Nucleosides, and Nucleobases: Analysis of Biological Samples in Health and Disease. Journal of Chromatography B: Biomed and Science Applications. (1993) 3–14.
- Yang FQ, Li DQ, Feng K, Hu DJ, Li SP. Determination of Nucleotides, Nucleosides and Their Transformation Products in Cordyceps by Ion-Pairing Reversed-Phase Liquid Chromatography-mass spectrometry. J. Chrom A. (2010). 5501–5510.
- Smith K, Rainville P. Utilization of MaxPeak High Performance Surfaces and the Atlantis Premier BEH C18 AX Column to Increase Sensitivity of LC-MS Analysis. Waters Application Note, 720006745, 2020.
- Walter TH, Alden BA, Belanger JL, Berthelette K, Boissel C, Delano M, Kizekai L, Nguyen JM, Shiner SJ. Modifying the Metal Surfaces in HPLC Systems and Columns to Prevent Analyte Adsorption and Other Deleterious Effects. LCGC (June 2022) 28-34. Accessed 6-July-2022 https://www.chromatographyonline.com/view/modifying-the-metal-surfaces-in-hplc-systems-and-columns-to-prevent-analyte-adsorption-and-other-deleterious-effects.
720007704, August 2022