This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the sensitivity benefits of the off-axis StepWave ion transfer technology in the SYNAPT G2-S provides organizations with the extended capability for intact biotherapeutic characterization.
Dig deeper into the character of biomolecules with UPLC and StepWave Technology in the SYNAPT G2-S System.
Analytical solutions that are able to provide more detailed characterization about a biomolecule (destined to become a biotherapeutic) have always been sought after by the biopharmaceutical industry. The incorporation of StepWave into a highperformance SYNAPT G2 High Defintion Mass Spectrometry System is another key step forward in achieving more detailed characterization of such molecules.
SYNAPT G2-S is equipped with a larger ion sampling orifice, an enhanced vacuum pumping configuration, and revolutionary StepWave ion transfer optics. This groundbreaking dual- T-Wave, off-axis design transfers ions from the ion source to the quadrupole MS analyzer with the highest possible efficiency, and at the same time, ensures that undesirable neutral contaminants are actively filtered out. This dramatically increases MS ion intensities while minimizing background noise – significantly improving detection limits and the repeatability of quantitative assays.
This application brief describes the performance characteristics of the UPLC-SYNAPT G2-S System for intact mass analysis of a therapeutic monoclonal antibody and its subunit (the light chain). The data will demonstrate the superior MS sensitivity and resolution that SYNAPT G2-S offers for a rapid, in-depth characterization of proteins.

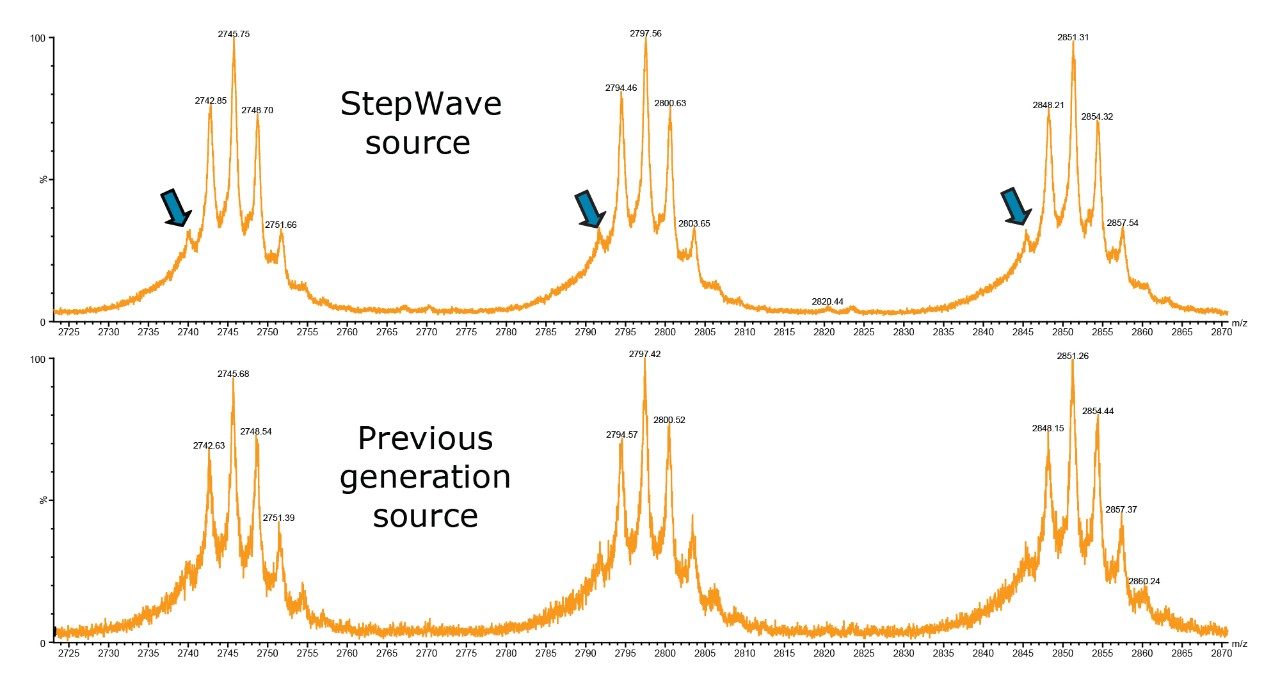
An experiment was performed to determine the effect of incorporating StepWave ion optics technology on the spectral data obtained when performing the intact mass analysis of an intact IgG1 monoclonal antibody (mAb), Trastuzumab. Data presented in Figure 1 were obtained during back-to-back experiments performed using two HDMS-enabled SYNAPT instruments, one of which utilized StepWave ion transfer optics (top). The UPLC-MS analysis of 1.0 μg intact Trastuzumab was acquired in the sensitivity mode of each SYNAPT instrument. Under equal sample loading, low abundance, minor glycoforms of the mAb molecule are better defined in the spectrum from the SYNAPT G2-S instrument (with StepWave), thus providing more comprehensive data about the glycoform distribution in this batch of therpeutic protein.
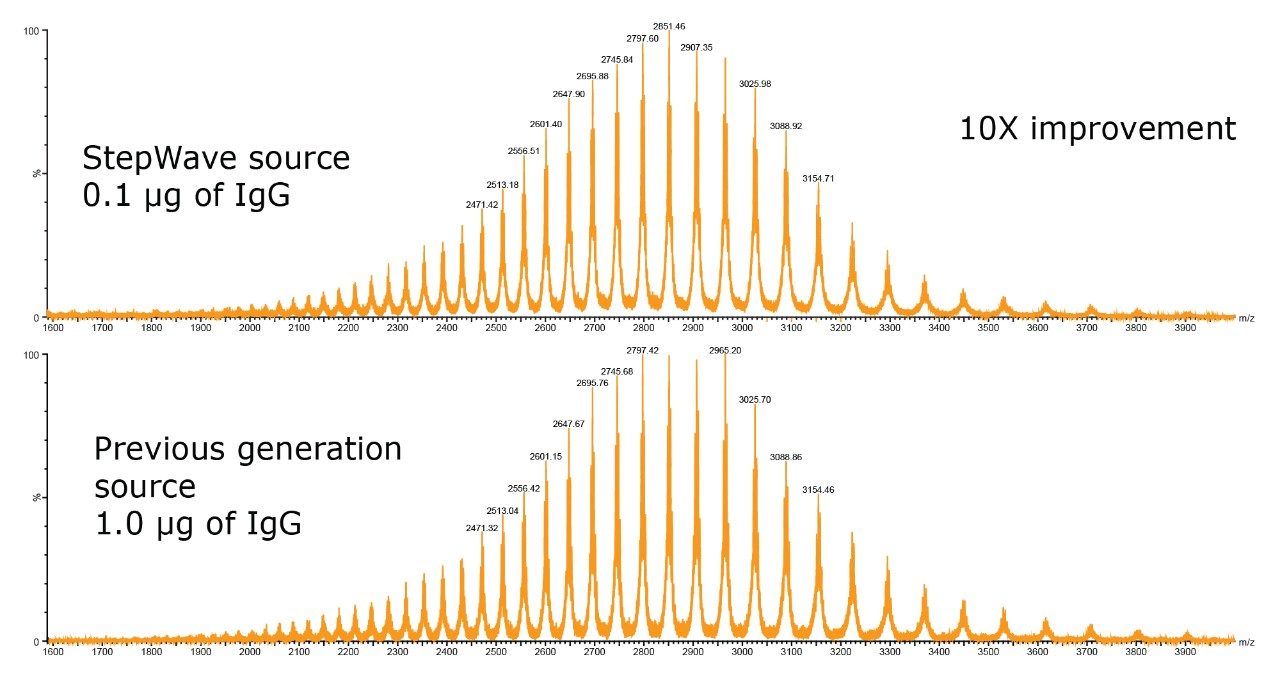
Increasing ion currents entering the SYNAPT G2-S mass spectrometer enables high quality spectra to be acquired with less sample consumption. Parallel LC-MS analyses for intact Trastuzumab were undertaken with the SYNAPT G2-S (shown in Figure 2, top) and SYNAPT G2 (shown in Figure 2, bottom). Similar spectra were generated by the two instruments, even though 10-fold less material was loaded on column for the SYNAPT G2-S System, thus demonstrating the increased sensitivity of the instrument enabled with StepWave technology.
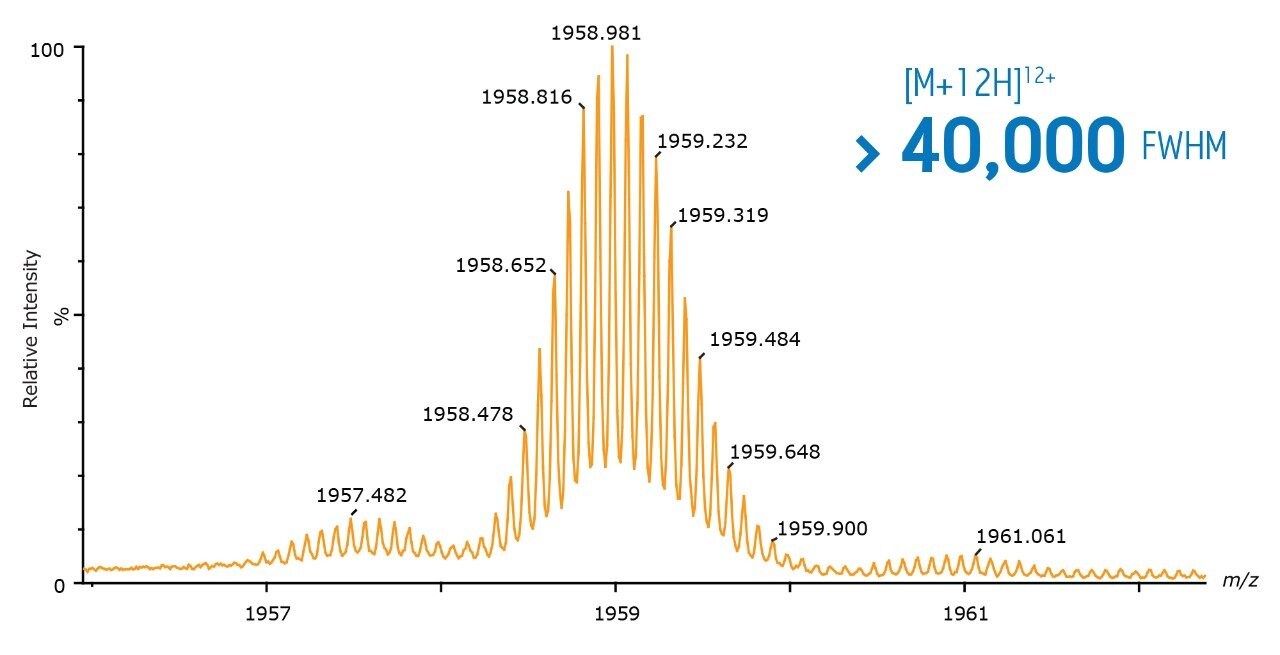
The SYNAPT G2-S is capable of achieving isotopic resolution of proteins with a size of 30 kDa and yielding low ppm mass accuracy results under routine LC-MS conditions. The clearly defined isotopic pattern of the 25 kDa Trastuzumab light chain subunit enables isotopic models to be applied for the detection of lower mass modifications, thus providing sensitive characterization with greater structural detail from the intact protein analysis.
720004003, June 2011