In this study, an optimized combination of Waters technologies that involves best-in-class sample preparation chemistry, UPLC chromatography, and the Xevo TQ-S tandem quadrupole mass spectrometer allows determination of ethinyl estradiol at its LLOQ of 1 pg/mL in plasma.
Ethinyl estradiol is present in very low concentration in human blood. The ability to detect it at low concentration is an important challenge for the analytical techniques that are used in the today’s world of bioanalysis. In this study, an optimized combination of Waters technologies that involves best-in-class sample preparation chemistry, UPLC chromatography, and the Xevo TQ-S tandem quadrupole mass spectrometer allows determination of ethinyl estradiol at its LLOQ of 1 pg/mL.
Ethinyl estradiol is an orally bioactive estrogen used in almost all modern formulations of combined oral contraceptive pills. Ethinyl estradiol is a chemical analogue of estradiol, formed by substitution at the C17 carbon with an ethinyl group. This substitution significantly reduces ethinyl estradiol’s susceptibility to metabolism in the liver, compared to estradiol, allowing it to be employed as a low-dose, once-a-day oral contraceptive.
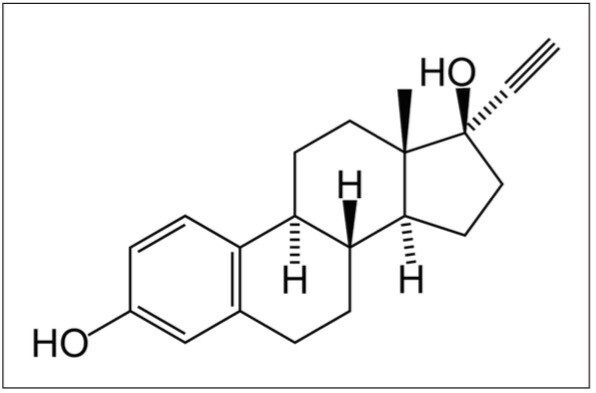
Ethinyl estradiol is readily absorbed in gut following oral administration with a maximum concentration occurring 2 hours after dosing. It is cleared by extensive metabolism in the liver by the cytochrome p450 3A4 isoenzyme and excreted in the bile.
The compound then undergoes enterohepatic recirculation, which results in a second plasma concentration peak several hours later. The major metabolite is hydroxylation of an aromatic ring followed by glucuronidation or sulphation, which are eliminated in feces and urine. As the compound is extensively metabolized and heavily protein-bound, the circulating levels of ethinyl estradiol are very low; hence, a high-sensitivity method is required to accurately quantify the compound during dose formulation and bioequivalence studies
The samples were isolated using solid phase extraction employing a Waters Oasis MCX (1 mL, 30 mg) cartridge. A 300-μL aliquot of plasma was diluted with an aqueous buffer solution and loaded onto the SPE cartridge previously conditioned with organic solvent and water. The plasma solution was then washed with an organoaqueous solution followed by water. The samples were eluted with organic solvent eluted samples, evaporated to dryness, and reconstituted in buffered solvent, then derivatized with dansyl chloride heated to 60 °C followed by analysis by LC-MS/MS.
The extracted samples were analyzed by reversed-phase isocratic chromatography employing an acidic aqueous buffer and acetonitrile as the organic modifier.
|
LC system: |
ACQUITY UPLC System equipped with a Binary Solvent Manager, Column Manager, and Sample Manager |
|
LC column: |
ACQUITY UPLC HSS T3 C18 1.8-μm, 2.1 x 100 mm |
|
Elution: |
80% organic acidic 20% aqueous buffer over 6 minutes followed by a high concentration organic wash |
|
Column temp.: |
45 °C |
|
MS system: |
Xevo TQ-S |
|
MS mode: |
Positive ion electrospray MS/MS |
|
MS transition : |
530 ⇒ 171 |
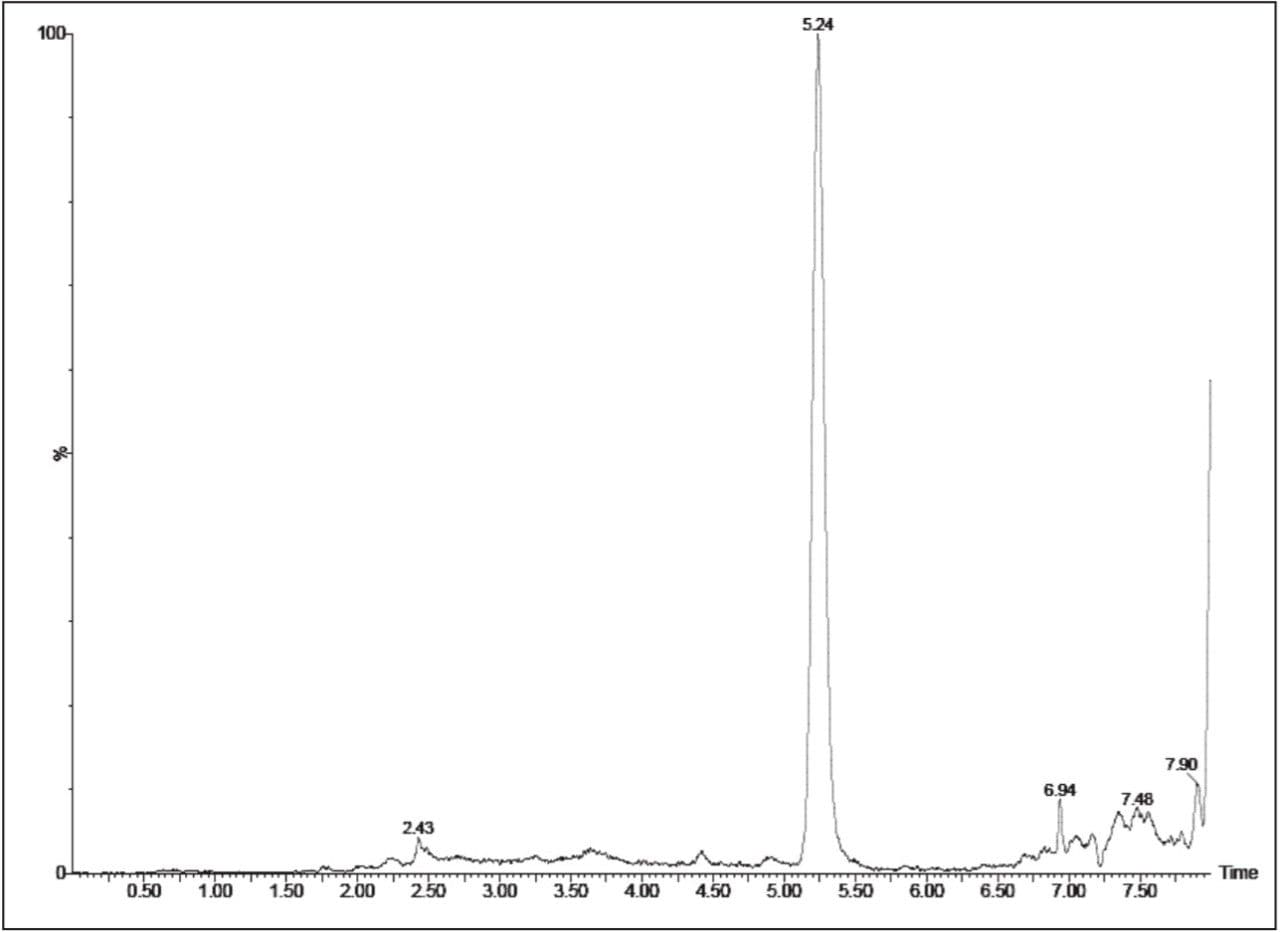
The chromatographic method allowed for the excellent resolution of the ethinyl estradiol analyte from the endogenous components in the samples. As can be seen in Figure 2, the target analyte elutes with a retention time of 5.24 minutes. The high-resolution separation was facilitated by the high-resolution performance of the ACQUITY UPLC System and T3 HSS 1.8-μm column.
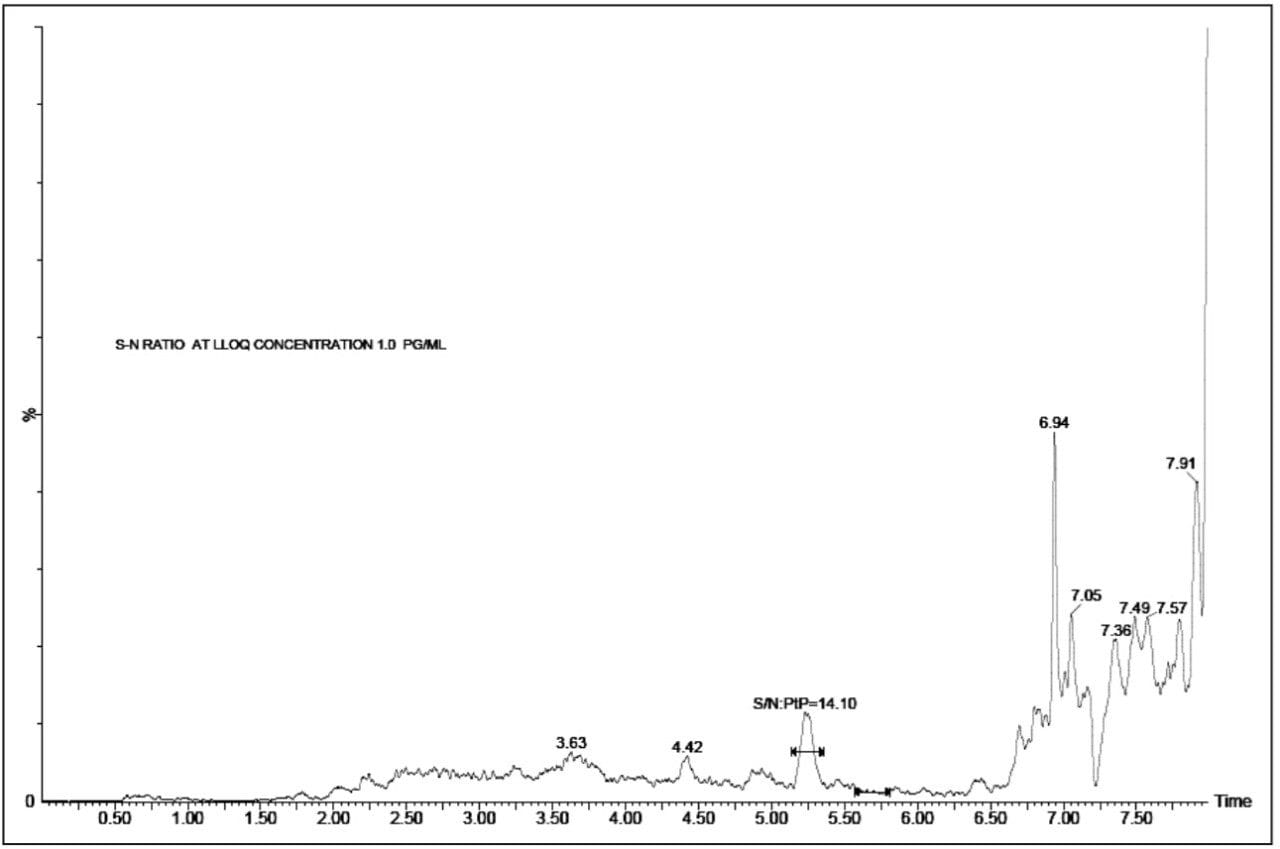
The Xevo TQ-S mass spectrometer is equipped with a novel StepWave ion guide that enables improved ion sampling in the source and ion transfer efficiency. This new ion guide optics, when combined with the high-resolution chromatography produced by the ACQUITY UPLC System, results in a lower limit of quantification of 1 pg/mL, Figure 3. In this example we can see that the system develops a signal-to-noise (S/N) value of 14:1.
The high sensitivity achieved for this assay was due to the combination of selective extraction, high-resolution chromatography provided by ACQUITY UPLC, and the sensitivity of the Xevo TQ-S. The new Q0 region in the Xevo TQ-S, fitted with the unique StepWave Technology, allows a significantly greater amount of the ion flux to be sampled without contaminating the instrument source region.
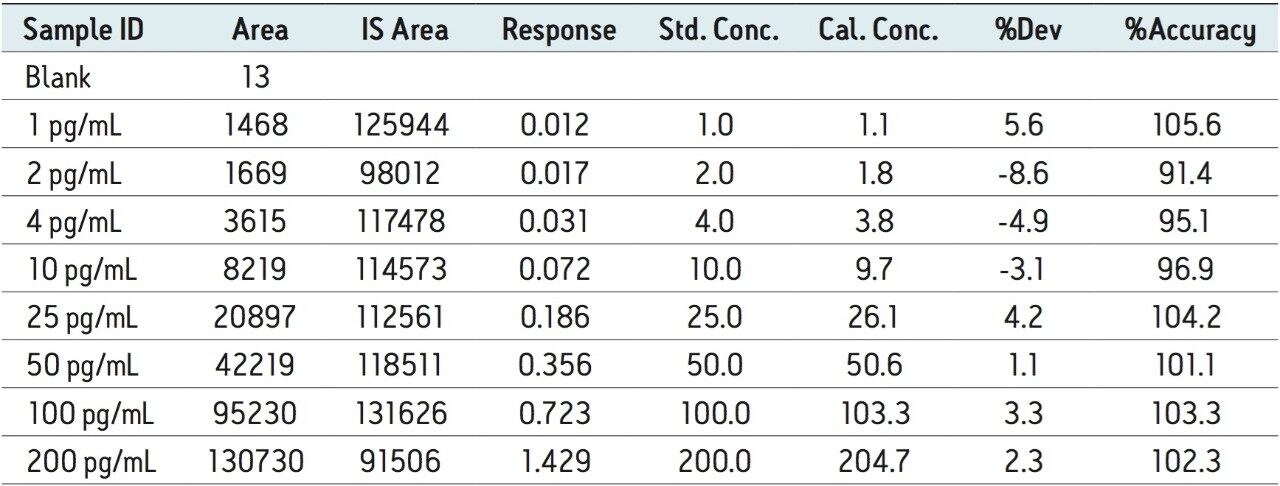
The method delivered a linear calibration response over the range of 1 to 200 pg/mL with a correlation coefficient of 0.996642. The data displayed in Table 1 shows the back-calculated concentrations of the standards. From this we can see that the variation was only +/- 9%, showing that the method had excellent linearity and precision.
Ethinyl estradiol, which is commonly used as an oral contraceptive, is present in very low concentration in human blood. The ability to detect it at such low concentration is an important challenge for the analytical techniques that are used in the today’s world of bioanalysis. In this study, an optimized combination of Waters technologies that involves best-in-class sample preparation chemistry, ACQUITY UPLC, and the Xevo TQ-S tandem quadrupole mass spectrometer allows determination of ethinyl estradiol at its LLOQ of 1 pg/mL.
A high-sensitivity assay for the quantification of ethinyl estradiol in plasma was developed with a LLOQ of 1 pg/mL. The assay was shown to be linear over the range of 1 to 200 pg/mL. The high sensitivity and linearity of the was due to the extraction specificity of the Oasis MCX solid phase extraction system, the high resolution of the ACQUITY UPLC System and the sensitivity of the Xevo TQ-S Mass Spectrometer. The LLOQ of this method, of 1 pg/mL, would allow the accurate determination of ethinyl estradiol in plasma.
720004058, August 2011