
In this application note, we compare the use of fluorinated-phenyl phases, including the ACQUITY UPLC HSS PFP and ACQUITY UPLC CSH Fluoro-Phenyl Columns, with C18 and Phenyl stationary phases. For method development, column screening is facilitated with automation, using the ACQUITY UPLC H-Class System with a four-position Column Manager.
During method development, the selection of the most appropriate column for the tested analyte should always be considered. Often, a C18 column is chosen due to its versatility and common availability in most laboratories. For many separations, this column may be sufficient. However, for certain samples, columns that employ a different retention mechanism and diverse selectivity may be quite useful in obtaining the desired separation. Understanding the benefits of using columns, such as those with fluorinated-phenyl stationary phases, facilitates selection of the best column for the sample in the early stages of method development, thereby reducing the need for lengthy downstream optimization of the separation.
In this application note, fluorinated-phenyl phases including the ACQUITY UPLC HSS PFP and ACQUITY UPLC CSH Fluoro-Phenyl Columns will be compared to both C18 and Phenyl stationary phases. The comparisons are performed on a variety of samples to demonstrate the alternate selectivity of these columns for analytes composed of different chemical properties. By correlating the sample properties to the column chemistries, a more ideal column selection and better separation can be achieved earlier, enabling a faster, more efficient method development process.
|
System: |
ACQUITY UPLC H-Class |
|
Mobile phase: |
A: water with 0.1% formic acid, B: acetonitrile with 0.1% formic acid |
|
Gradient: |
2% to 98% B over 8 min, hold for 1 min, re-equilibrate at 2% B |
|
Detection: |
UV at 254 nm (for all, except paroxetine at 295 nm) |
|
Needle wash: |
90:10 acetonitrile/water |
|
Sample purge: |
90:10 water/acetonitrile |
|
Seal wash: |
50:50 methanol/water |
|
Flow rate: |
0.4 mL/min |
|
Column temp.: |
30 °C |
|
Injection volume: |
2 μL |
|
Vials: |
TruView Maximum Recovery Vials, p/n 186005662CV |
|
Columns: |
ACQUITY UPLC 2.1 x 100 mm, 1.7 to 1.8 μm |
|
Stationary phases: |
ACQUITY UPLC CSH C18, p/n 186005297 ACQUITY UPLC CSH Phenyl-Hexyl, p/n 186005407 ACQUITY UPLC CSH Fluoro-Phenyl, p/n 186005352 ACQUITY UPLC HSS PFP, p/n 186005967 |
|
Data Management: |
Empower 3 CDS |
USP standards of paroxetine and related compounds B, D, and F were prepared at concentrations of 0.2 mg/mL (paroxetine), and 0.02 mg/mL (paroxetine related compounds) in 50:50 methanol/water, and transferred to a TruView Maximum Recovery Vial for injection.
Amcinonide base degradation: 1 mg/mL of amcinonide standard was prepared in sample diluent (80% methanol in water), was reacted with 1 N NaOH (1:1), and stirred at 60 °C for 30 minutes. The reaction was neutralized with HCl, diluted to a final concentration of 0.25 mg/mL with sample diluents, and transferred to a TruView Maximum Recovery Vial for injection.
Flavone and flavanone: Flavone and flavanone standards were prepared at 1 mg/mL in methanol and reacted with 1 N NaOH (1:1). The reaction was stirred at room temperature for 1 hour, treated with 1 N HCl, diluted to a final concentration of 0.125 mg/mL with methanol, and transferred to a TruView Maximum Recovery Vial for injection.
Famotidine acid degradation: A 10-mg tablet sample of famotidine was dissolved in 50:50 methanol/water to a concentration of 1 mg/mL. 1 N HCl (1:1) was added, and the sample was heated at 60 °C and stirred for 2 hours. The sample was then neutralized with 1 N NaOH, diluted to a final concentration of 0.25 mg/mL, and transferred to a TruView Maximum Recovery Vial for injection.
In many labs, a C18 column is commonly used as a starting point in chromatographic method development. Although a C18 column is very versatile, it does not always provide the best separation, depending on the sample matrix and analyte of interest. In cases of critical separations between closely eluting compounds, using columns with very different selectivity (as identified by the Waters Column Selectivity Chart, www.waters.com/selectivitychart) and alternate retention mechanisms can quickly provide the desired separations. In this application, column screening was facilitated with automation, using the ACQUITY UPLC H-Class System with a four-position Column Manager. The columns that were tested could easily be tracked during data analysis using the ACQUITY UPLC Column eCord and Empower 3 Software. Selectivity changes were monitored by peak tracking using reference standards and UV spectra profiles.
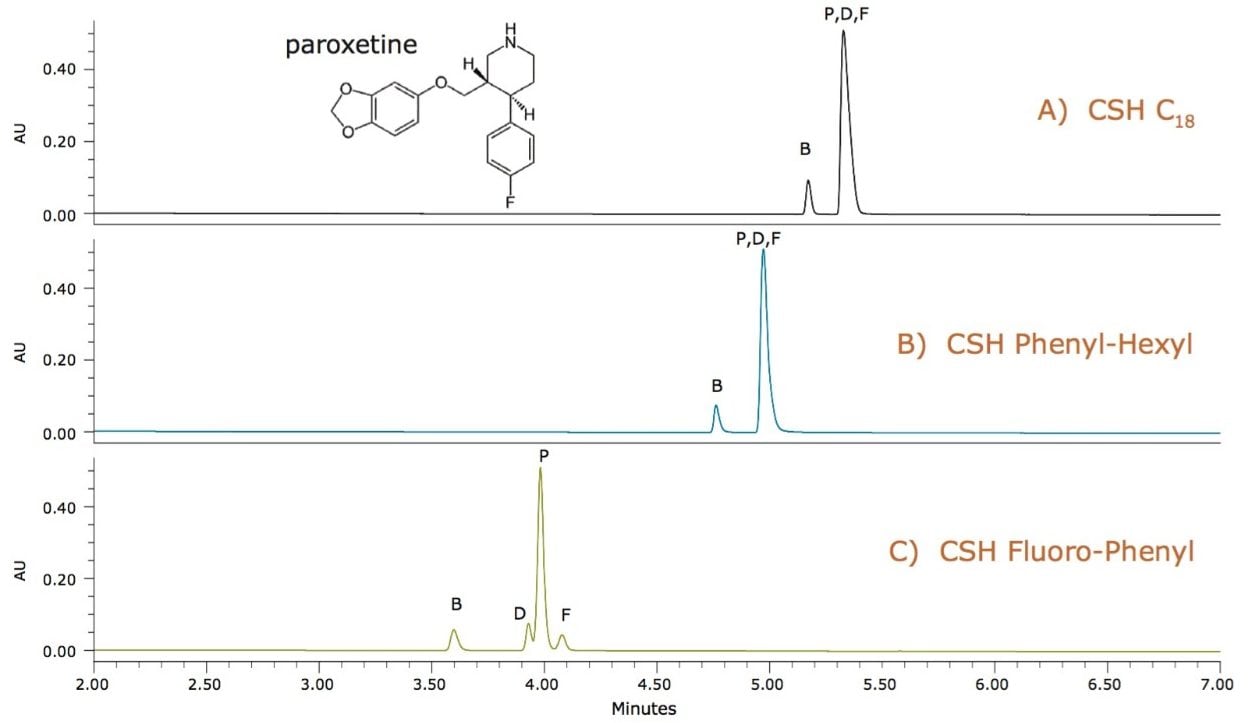
Paroxetine is a basic drug that has several aromatic groups and a halogenated functional group. The separation of paroxetine from its related compounds (B, D, and F) was first screened on a CSH C18 column, but the related compounds were not resolved, as shown in Figure 1A. Next, a CSH Phenyl-Hexyl column was tested to take advantage of retention due to π- π interactions between the phenyl groups on the stationary phase and aromatic groups on the analyte.1
However, this was still insufficient to achieve the desired resolution, as seen in Figure 1B. A fluorinated-phenyl phase takes advantage of π- π interactions, but also has altered electron density around the fluorinated-phenyl ring, resulting in different charge transfer and electrostatic interactions between the analyte and the stationary phase.2 In addition, the fluorinated-phenyl ring is larger than the phenyl alone, possibly resulting in altered retention profiles due to steric effects. For paroxetine, a quick screen of the CSH Fluoro-Phenyl column resolved the three related compounds from the paroxetine API, as shown in Figure 1C, resulting in a much simpler optimization of the method during development.
For compounds containing aromatic groups, differences in retention mechanisms between phenyl-based ligands, such as the CSH Phenyl-Hexyl and Fluoro-Phenyl columns, can also result in very different selectivity for a separation. In Figure 2, the separation of flavanone and flavone degradation products is compared on the two aromatic stationary phases. Alternate retention mechanisms, including electrostatic or steric interactions using the Fluoro-Phenyl phase, can result in a shift in elution order. In this example, a better overall separation is achieved on the Phenyl-Hexyl phase, demonstrating the utility of screening both types of phenyl-based ligand columns to maximize selectivity differences and quickly identify desirable separations early in the method development process.
![Changes in selectivity for the separation of aromatic compounds [flavone (A), flavanone (B), and flavanone base degradation products (C, D)] on phenyl-based CSH stationary phases.](/content/dam/waters/en/app-notes/2012/720004485/720004485en-f2.jpg.82.11-15-1269-574C.resize/img.jpg)
In addition to retention due to the ligand, the chemistry of the base particle can also affect retention, offering different selectivity of the separation. For the separation of amcinonide base degradation products, two columns with the same fluorinated-phenyl ligand were tested, as shown in Figure 3. In this example, the charged surface hybrid (CSH) particle shows peaks A and B to be closely eluting. Using the silica-based HSS particle, the resolution of peaks A and B improves significantly, with a much better overall separation of components. Since the fluorinated-phenyl ligands are both the same, the difference in separation can be attributed mainly to the difference in the properties of the base particles (charged hybrid versus non-endcapped silica), with minor contribution from differences in particle pore size and ligand loading.

It is also important to note that, while PFP is the ligand for both particles, the difference in base particle properties can have a great impact on the retention of ionizable analytes. For instance, degradation products of famotidine, a basic compound, are far less retained on the CSH Fluoro-Phenyl column compared to the HSS PFP column. At low pH, charge repulsion between the positively charged famotidine-related analytes and the CSH stationary phase result in reduced retention. The lack of charge on the HSS base particle shifts the mode of retention toward the ligand properties rather than the particle surface interactions, resulting in a more typical PFP column behavior with greater retention for basic compounds. For ionizable analytes, the results are very different retention profiles, and potential selectivity differences between the two fluorinated-phenyl columns.
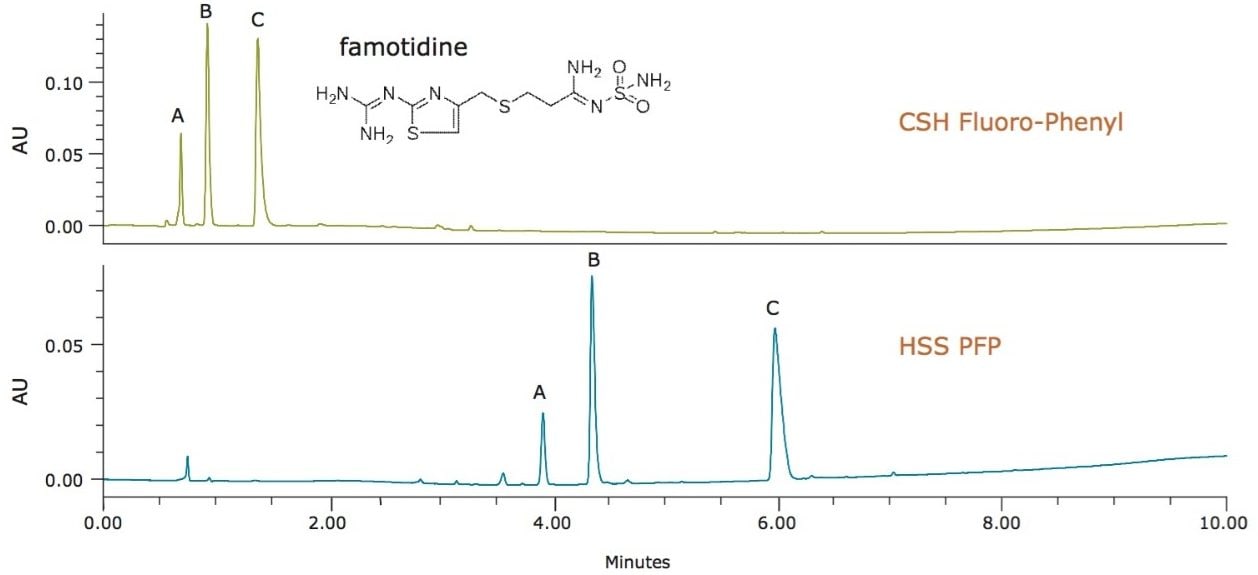
For separations where traditional C18 ligands are not ideal, it is appropriate to screen columns with alternate selectivity, such as fluorinated-phenyl stationary phases. This is especially true when the analyte is known to contain halogenated or aromatic functionalities. For compounds that are ionizable, the choice of base particle is particularly important to consider when selecting fluorinated-phenyl columns. Retention can be vastly different for ionizable compounds on a charged-surface hybrid (CSH) particle column compared to an HSS silica-based column, even with the same fluorinated-phenyl ligand. Screening columns with wide selectivity ranges, understanding interactions between the analytes and stationary phases, and early identification of the best column for a desired separation can save a tremendous amount of time and effort when developing a new method.
720004485, November 2012