Automating Preparation of Matrix-Matched Standards for Pesticide Residue Analysis Using the Andrew+ Pipetting Robot
This is an Application Brief and does not contain a detailed Experimental section.
Want to learn more about the Andrew+ Pipetting Robot?
Abstract
The Andrew+ Pipetting Robot and the cloud-native OneLab Software Platform have been successfully evaluated for automating the preparation of matrix-matched standards for pesticide residue analysis by LC-MS/MS. We found its performance consistent with the requirements associated with the preparation of standard solutions, even in a matrix extract. The characteristics of the calibration graphs were improved when matrix-matched standards were prepared automatically. Automating this important analytical step saves time, reduces risk of repetitive strain injury, minimizes opportunity for error, and enables technical staff to be freed up for other tasks, all while ensuring full traceability.
Benefits
- Fully automated process which can be completed, unattended in less than 14 minutes
- Easy to use with minimal training required
- Consistent pipetting results provides accurate and precise quantification
- Reduces the need for repetitive pipetting which can lead to repetitive strain injury
Introduction
Plant protection products, more commonly referred to as pesticides, are widely used across the globe to increase agricultural yields. Countries typically have an obligation to carry out controls to ensure that food placed on the market is compliant with the legal limits for pesticide residues. Compliance with such regulations is checked using official control programs at the national or regional level supported by increased frequency of import controls and testing undertaken by the food industry. There are various approaches available to analyze agricultural commodities, products of animal origin, and finished food products for pesticide residues, using multi-residue methods, such as the Dutch mini-Luke, Swedish ethyl acetate and Quick, Easy, Cheap, Effective, Rugged, Safe (QuEChERS), supported by a limited number of single residue methods.1 Cleanup is often omitted from the multi-residue methods so matrix effects are a common concern, which can compromise detection and quantification of pesticide residues when using either LC-MS/MS or GC-MS/MS. One mitigating solution is to prepare all the standard solutions in suitable volumes of a blank extract of the commodity being analyzed: matrix-matched calibration. This process is an essential part of any quantitative residue testing and needs to be carried out in an accurate, precise, and traceable manner. Automating this process would therefore minimize error, as well as reducing risk of injury (repetitive strain injury from pipetting) and allow the lab analyst to be deployed for other tasks.
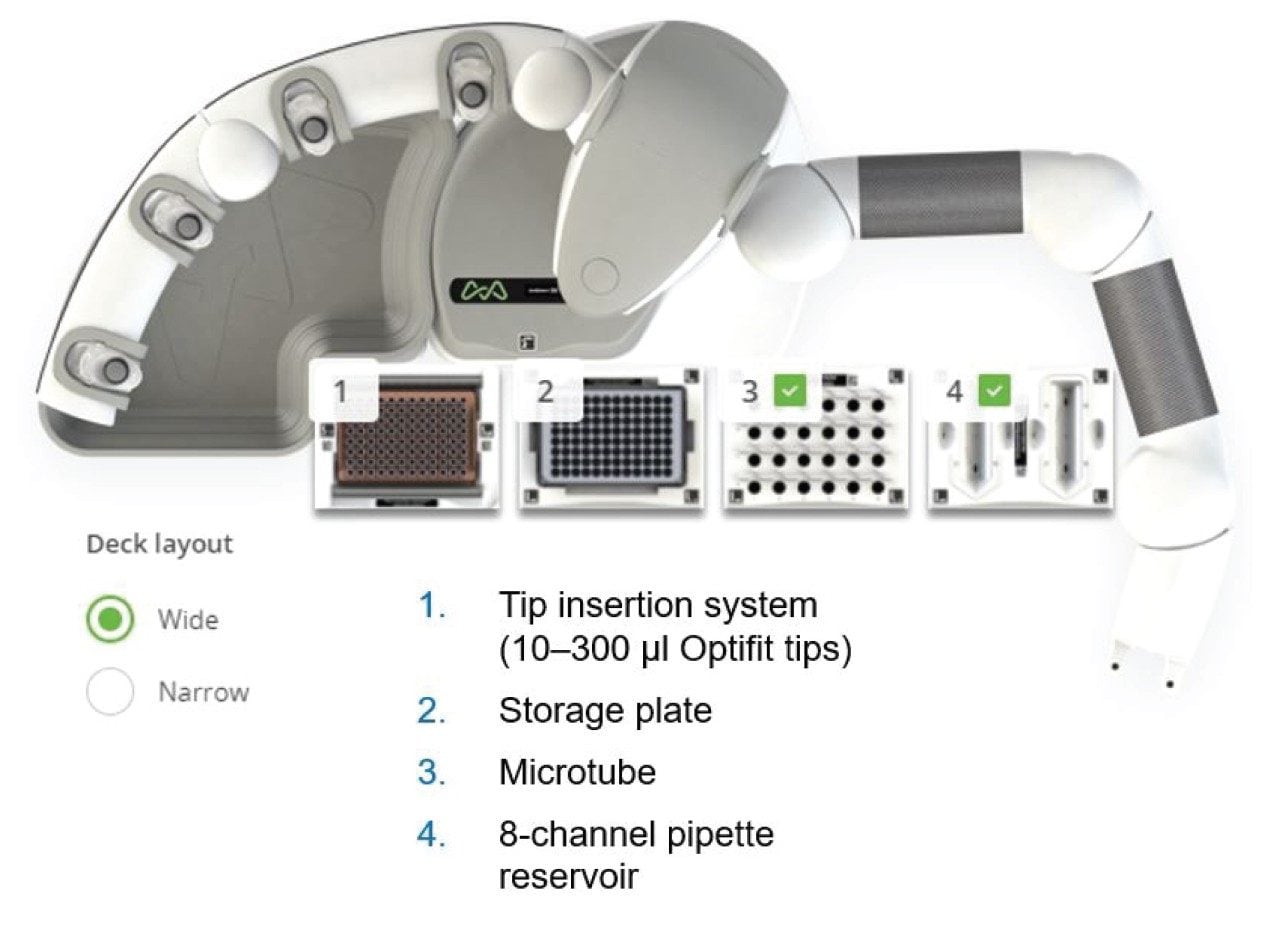
The Andrew+ Pipetting Robot offers fully automated pipetting, as well as more complex manipulations supported by a wide range of Domino Accessories and Andrew Alliance electronic pipettes. It executes OneLab protocols, enabling rapid transition from laborious manual procedures to error-free, robotic workflows. The platform is easy to set up and does not occupy much lab space. Even with 2 full rows of Dominos (allowing to use 7 microplates or 56 falcon tubes or 168 microtubes), the robot only occupies a depth of ~60cm. A previous white paper describes how innovations in automated liquid handling technology have lowered the barriers to adoption and shares the process in trialing an automation system to improve laboratory efficiency.2
Here, we present the first of two different approaches to automating the preparation of a set of matrix-matched calibrants for pesticide residue analysis, with a brief evaluation in the use of the Andrew+ Pipetting Robot controlled by OneLab Software.
Results and Discussion
The evaluation was focused on automating the preparation of a series of matrix-matched standard solutions, at five levels, for 20 representative pesticides in a QuEChERS extract of apple, analyzed using a typical LC-MS/MS method.3 Standards were prepared in duplicate in water/acetonitrile (9:1) to match the initial mobile phase conditions for improved chromatographic peak shape of early eluting compounds. The two sets of standards were then analyzed to create a bracketed calibration from the data. The scope of the evaluation was designed to assess the performance of the pipetting robot using the calibration graph characteristics as benchmark but also to determine the time taken for this task. The results are then compared with repeating the process manually by an experienced analyst.
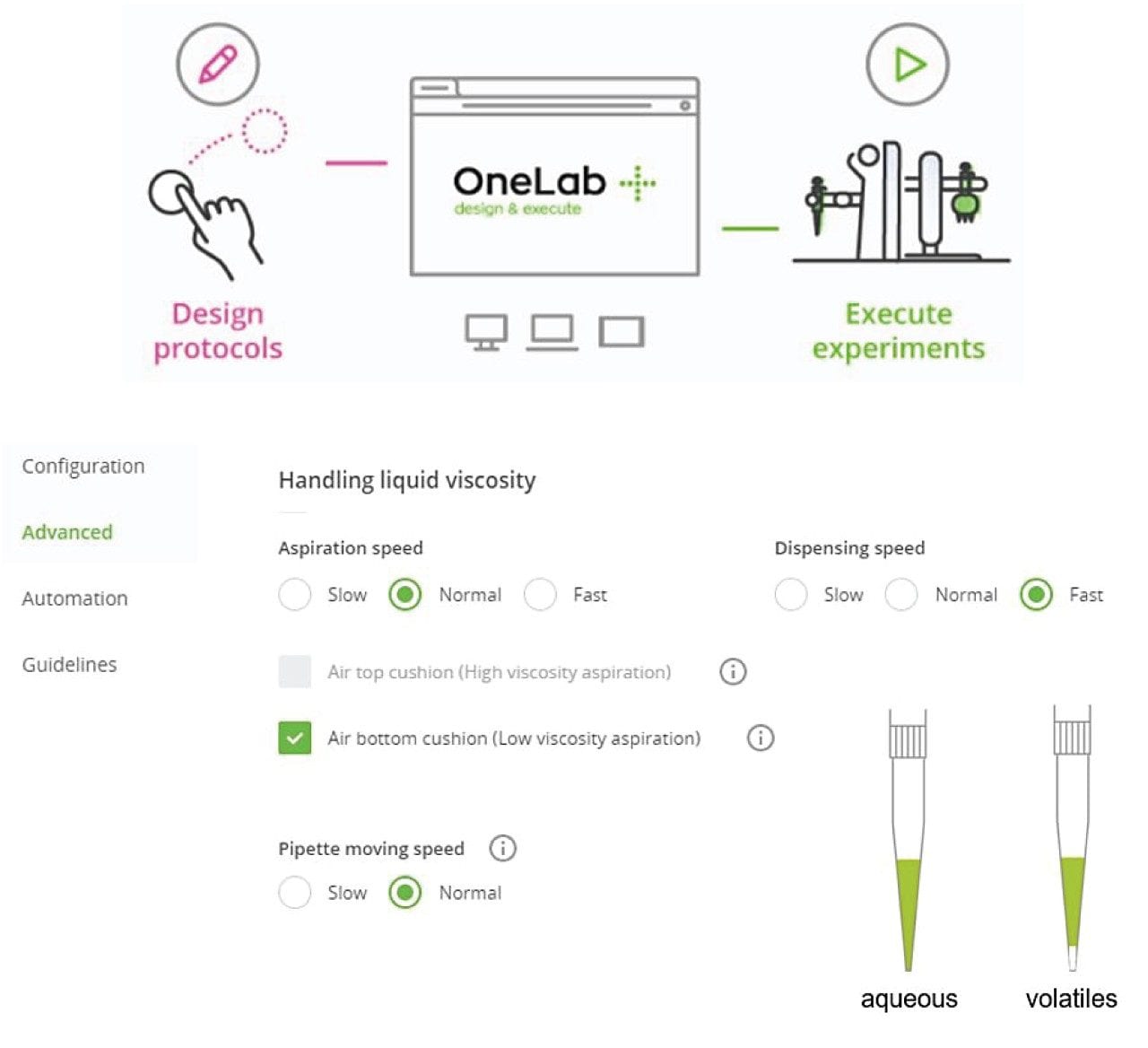
During the evaluation, the OneLab Software user interface has an intuitive graphical design; it took under 30 minutes to design the pipetting protocol, which then could be executed as and when required with no further programming. OneLab Software allows users to trace all steps from experimental design through to the protocol execution. Settings can be customized to meet specific experimental needs. For example, aqueous and organic solvents require different aspiration speeds. When handling organic solvents such as acetonitrile, the user has the option of using “air bottom cushion” to prevent dripping. The automation platform provided the additional benefit of traceability as the operation steps were recorded as scripts so it was possible to check exactly how the standards were prepared in case troubleshooting should be required.
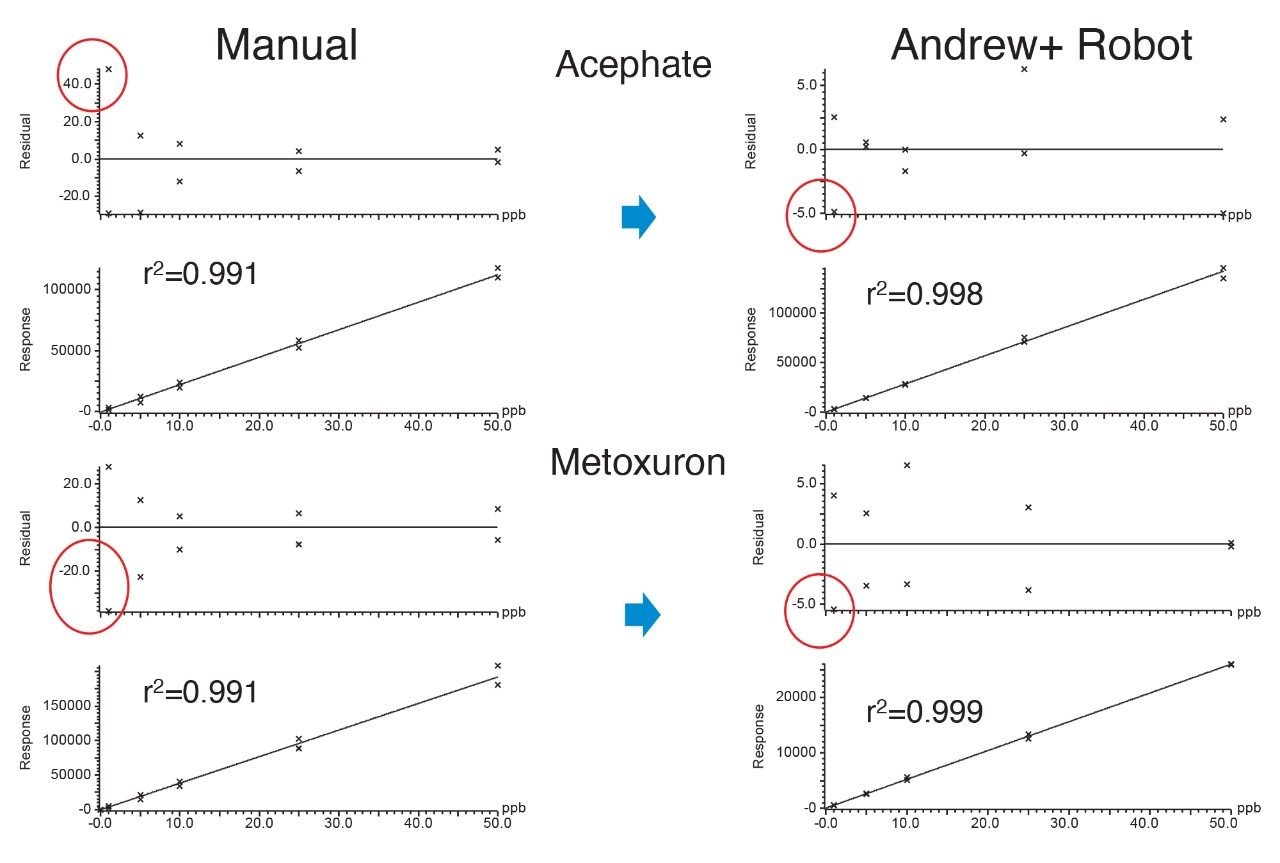
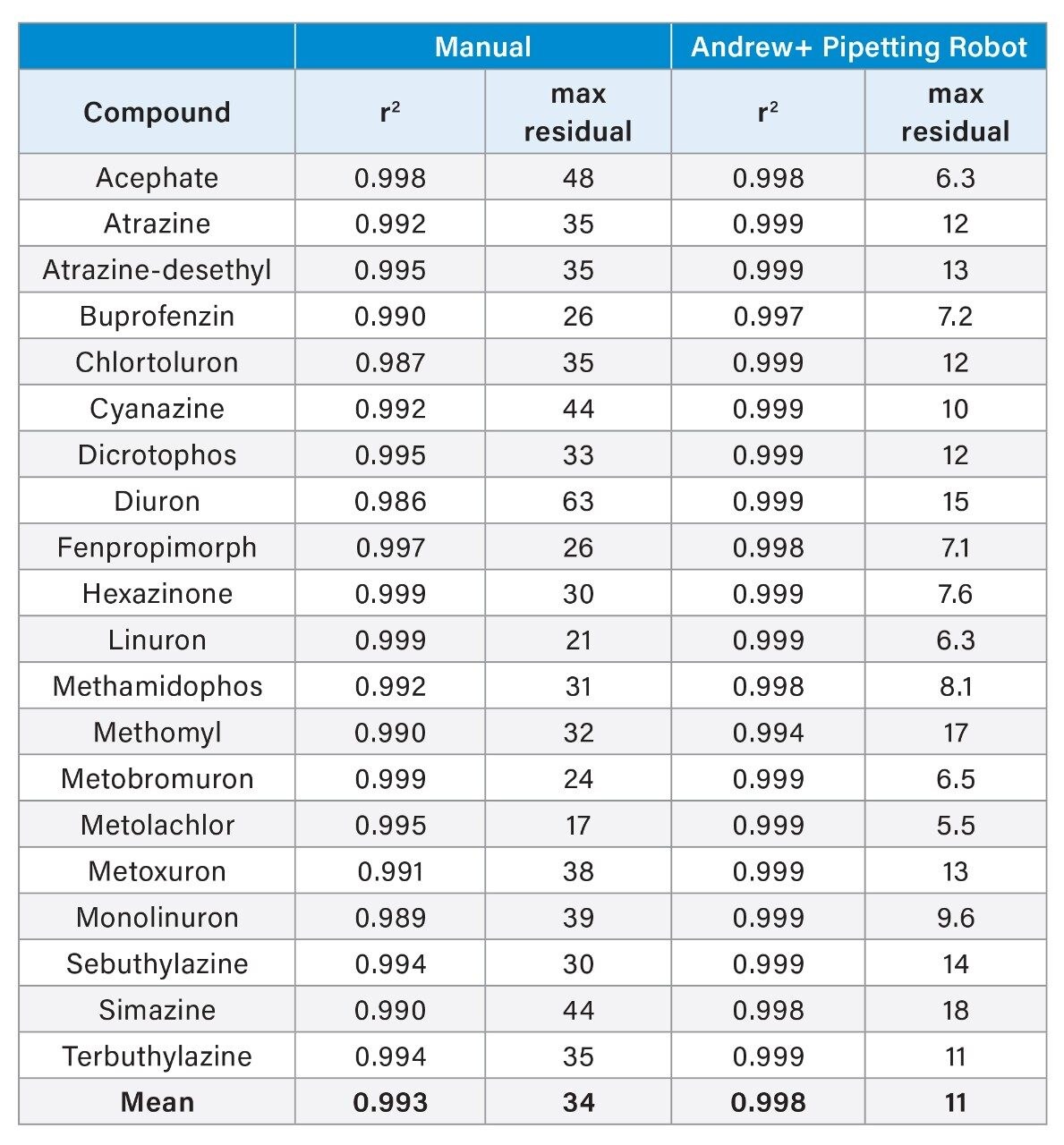
Calibration graphs were created from the analysis of each of the duplicate sets of matrix-matched standards, using a linear fit with a 1/x weighting. Acceptance criteria for quantification often cite values for coefficient of determination (r2) and residuals. A residual is the difference between the true value and the value predicted by the equation of the fitted line, which gives an indication of how well the line fits the data. There was no significant difference between the values for r2 for the graphs from standards prepared automatically and those from the manual procedure (r2 = 0.998 and 0.996, respectively). The residuals in the calibration graphs from analysis of the duplicates of the standards created by the Andrew+ Pipetting Robot shows an improvement when compared to those from the analysis of standard solutions created manually, especially at the lower concentrations. See Figure 3 for some examples of calibration graphs and Table 1 for a summary of their characteristics.
The Andrew+ Pipetting Robot completed the task of preparing the matrix-matched standards at six levels, in duplicate, in less than 14 minutes, without human intervention whereas it took just over 35 minutes to prepare the same series of standard solutions manually using a single channel pipette. Using the Andrew+ Pipetting Robot also reduces the potential of human errors resulting in the need to repeat the analysis.
Conclusion
The automated method developed on the Andrew+ Pipetting Robot has demonstrated comparable results to manual pipetting and is suitable for the preparation of matrix-matched standards for pesticide residue analysis using LC-MS/MS. Automation provides considerable time savings, as staff spend less time on tedious, repetitive manual tasks and it also removes the impact of human error that results in unwanted repeat analyses. Further work is planned to evaluate the accuracy and precision and to extend the scope to other solvent and commodity compositions.
References
- EURL-FV. Validation of MRM Pesticides from the Working Document SANCO/12745/2013 using Three Multiresidue Methods (QuEChERS, Swedish, ethyl acetate, and Dutch mini-Luke). 2019.
- Ross E, Meruva N M, Skinner N. Improving Method Reproducibility and Efficiency in Food Testing: How Can Liquid Handling Automation Help? Waters White Paper 720007332EN. August 2021.
- Shah D, Wood J, Fujimoto G, McCall E, Hird S, Hancock P. Multiresidue Method for the Quantification of Pesticides in Fruits, Vegetables, Cereals and Black Tea using UPLC-MS/MS. Waters Application Note 720006886EN. February 2021.
720007433, November 2021