Quantification of Thyroglobulin in Serum for Clinical Research Using SISCAPA™ Workflow Combined with LC-MS/MS
Apenas para utilização em pesquisas. Não se destina à utilização em procedimentos de diagnóstico.
Abstract
Thyroglobulin (Tg) is a 660 kDa homodimer synthesized by the follicular cells of the thyroid gland, acting as a substrate in the production of the hormones triiodothyronine (T3) and thyroxine (T4). Accurate measurement of Tg using existing immunoassay-based techniques can be challenging in the presence of anti-Tg antibodies (TgAb), which can prevent the binding of Tg to assay antibodies thus leading to non-quantifiable Tg concentrations. The Stable Isotope Standards and Capture with Anti-Peptide Antibodies (SISCAPA) workflow combined with liquid chromatography - tandem mass spectrometry (LC-MS/MS) has been successfully employed to circumvent this problem by digesting the serum sample thus eliminating interfering TgAb and measuring a Tg-specific surrogate peptide instead. Given the general complexity of this workflow it has been predominantly implemented in laboratories experienced in LC-MS/MS protein analysis. Here we report the development of a SISCAPA™ Workflow for accurate measurement of thyroglobulin on an Andrew+™ Pipetting Robot, enabling easier adoption of this workflow.
Briefly, a method was developed where serum Tg was digested and the signature peptide, FSPDDSAGASALLR (FSP), captured using SISCAPA Tg reagents (SISCAPA, Canada). Samples were prepared using both manual and automated protocols, with automation performed by the Andrew+ Pipetting Robot, which reduces the potential for user error, and improves laboratory efficiency by minimizing touch-time. Using an ACQUITY™ UPLC™ I-Class PLUS FL System, FSP peptide was chromatographically separated on a XSelect™ Premier HSS T3 Column and detected using multiple reacting monitoring (MRM) on a Xevo™ TQ Absolute Mass Spectrometer. The developed method was shown to be analytically sensitive and selective over the range of 0.1–50 ng/mL using 250 µL serum, with precision <9.5% CV across the concentration range.
Benefits
- Analytically sensitive analysis of thyroglobulin down to 0.1ng/mL using SISCAPA and conventional flow LC from 250 µL of serum
- Allows for re-injection of sample for repeat analysis
- Automation of the method to minimize touch-time and reduce potential for error
Introduction
Thyroglobulin (Tg) is a 660 kDa homodimer synthesized in the follicular cells of thyroid gland, acting as a precursor to the thyroid hormones, triiodothyronine (T3) and thyroxine (T4). Measurement of Tg has been traditionally performed using immunoassay-based techniques. However, both anti-thyroglobulin autoantibodies (TgAb) and heterophilic antibodies (HAbs) have been shown to interfere with Tg measurements on immunoassays, resulting in inaccurate results. In some instances, TgAb interference can result in a non-quantifiable measurement by immunoassay, when follow-up tests indicate the presence of a quantifiable concentration of thyroglobulin in the serum sample. The analytical variability observed with immunoassay can have a significant impact on the quality of clinical research studies, so it is evident that an alternative measurement technique is required in the presence of these antibodies.
The SISCAPA Workflow combined with liquid chromatography - tandem mass spectrometry (LC-MS/MS) has been shown to be an effective clinical research tool that circumvents the latter problem. In this workflow a proteotypic surrogate peptide unique to Tg is enriched and quantitated using mass spectrometry after the digestion of the serum sample, which completely eliminated protein-protein interactions between circulating Tg and TgAb or HAbs. The additional benefit of the SISCAPA Workflow is that low abundance proteins, such as Tg, can be quantified within the range of a typical MS/MS system even in presence of higher abundance proteins due to stoichiometric flattening dictated by the fact that the amount of antibody used in the workflow for each analyte can be adjusted accordingly. Inter-lab method comparisons have been performed for Tg, demonstrating excellent correlation between LC-MS/MS methods that target the Tg FSPDDSAGASALLR (FSP) signature peptide using immunoaffinity combined mass spectrometry.1 To further improve inter-lab performance, a distributable reference material for serum Tg was developed to assist with LC-MS/MS harmonization across laboratories.2
To demonstrate performance of a LC-MS/MS method for the analysis of serum Tg, we employed enzymatic digestion and SISCAPA Workflow of serum samples, targeting the FSP peptide, followed by injection on the ACQUITY UPLC I-Class PLUS FL System, with separation using the XSelect Premier HSS T3 Column and detection using the Xevo TQ Absolute Mass Spectrometer (Figure 1). The method was also automated on the Andrew+ Pipetting Robot to demonstrate equivalent performance to manual preparation, with the automation improving laboratory efficiency by minimizing touch-time.

Experimental
Sample Preparation
A 10 µg/mL thyroglobulin Certified Reference Material (CRM) (Merck, UK) solution was used to create calibrators in Tg negative surrogate serum matrix (chicken serum) (Merck, UK) over the range of 0.1-50 ng/mL. In-house Quality Controls (QCs) were prepared using pooled human serum (BioIVT, UK), which was value assigned at 15 ng/mL using in-house calibrators. This was diluted with surrogate serum to create pools at 0.3 and 3 ng/mL and supplemented with 20 ng/mL Tg CRM to create a high QC pool at 35 ng/mL. FSPDDSAGASALLR*(*13C6, 15N4) (Biosynth, USA) was used as the Stable Isotope Labelled (SIL) internal standard.
Thyroglobulin concentrations are typically reported in conventional mass units. To generate the same molar concentration of Tg FSP peptide in mass units, divide the thyroglobulin protein mass concentration by 234.8 (Tg monomer MW ≈ 330,000 Da, Tg FSP peptide MW = 1405.7 Da)
Samples were centrifuged at 3000 g for five minutes prior to use. 250 µL serum was transferred to a Axygen™ 1.1 mL 96-well collection plate.
Denaturation/Reduction: 100 µL of denaturant and reducing agent (10% (w/v) Deoxycholate, 0.015 M TCEP in 0.2 M Trizma™ Base) was added to each sample. Samples were mixed at 800rpm for 40 minutes at 37 °C. 30 µL of 0.05 ng/mL FSP-15N 13C6 SIL internal standard in 2% acetonitrile and 0.5% formic acid was added to each sample and mixed for 30 seconds.
Digestion/Quench: 50 µL of 10 mg/mL Trypsin in 0.2 M Trizma™ Base was added. Samples were mixed at 800 rpm for 30 minutes at 37 °C. 25 µL cOmplete™ Protease Inhibitor Cocktail(aq) was added to each sample and mixed for 10 minutes.
Capture: 25 µL of diluted SISCAPA Tg beads (1 in 2.5 dilution with 0.05% (w/v) CHAPS in PBS) were added to each sample and mixed briefly at 1100 rpm for 10 seconds, followed by 900 rpm mix for 30 minutes at room temperature. This was followed by another 1100 rpm mix for 10 seconds, followed by 900 rpm mix for 30 minutes at room temperature.
Wash: The collection plate was added to a magnetic array (SISCAPA, Canada), allowing the beads to pull down for two minutes. The entire liquid volume was removed and 250 µL of 0.05% (w/v) CHAPS in PBS was added to the sample and mixed for 1 minute at 1000 rpm. The plate was transferred back to the magnetic array and beads were left to pull down for 1 minute prior to wash removal. The wash step was repeated once.
Elution: 50 µL of 2% acetonitrile in 0.5% formic acid was added to each sample. The collection plate was mixed at 1100 rpm for 10 minutes. The plate was transferred back to the magnetic array and beads were left to pull down for one minute prior to elution transfer to a 700 µL Waters™ 96-well collection plate. The collection plate was sealed and an autosampler magnetic plate (SISCAPA, Canada) was secured to the collection prior to injection.
LC Conditions
|
LC system: |
ACQUITY UPLC I-Class PLUS FL System |
|
Detection: |
Xevo TQ Absolute Mass Spectrometer |
|
Plate: |
Waters 96-well Sample Collection Plate, 700µL |
|
Column(s): |
XSelect Premier HSS T3 Column, 100 Å, 2.1 mm x 50 mm, 2.5 µm |
|
Column temperature: |
45 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
20 µL |
|
Injection mode: |
Partial Loop |
|
Needle size: |
20 µL |
|
Sample syringe: |
250 µL |
|
Sample loop: |
50 µL |
|
Flow rate: |
Refer to Gradient Table |
|
Mobile phase A: |
0.01% formic acid in water |
|
Mobile phase B: |
Acetonitrile |
|
Weak wash: |
15% (v/v) acetonitrile in water |
|
Strong wash: |
25/25/25/25 (v/v) acetonitrile/methanol/IPA/water |
|
Run time: |
2.6 minutes |
MS Conditions
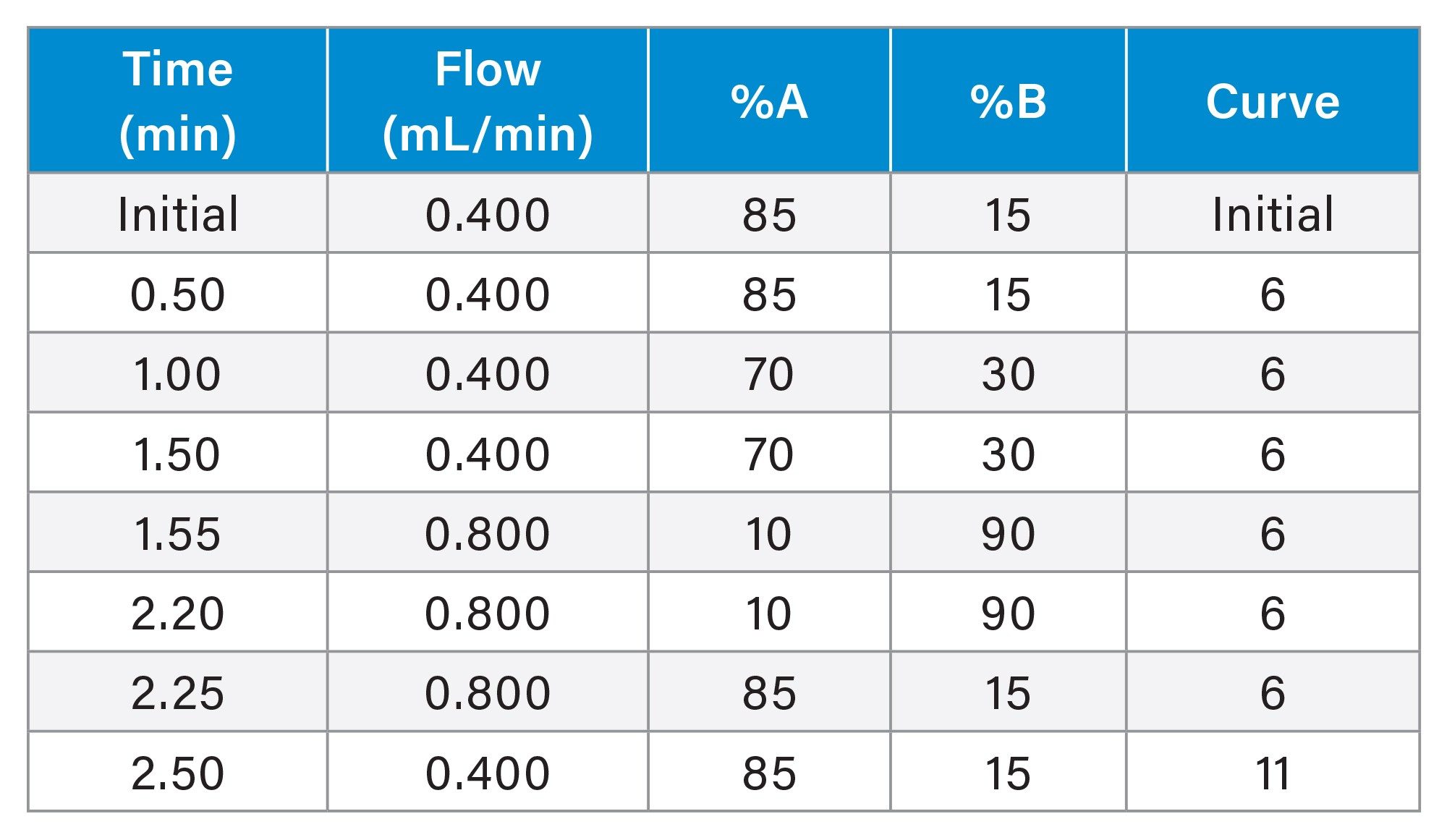
MRM Parameters
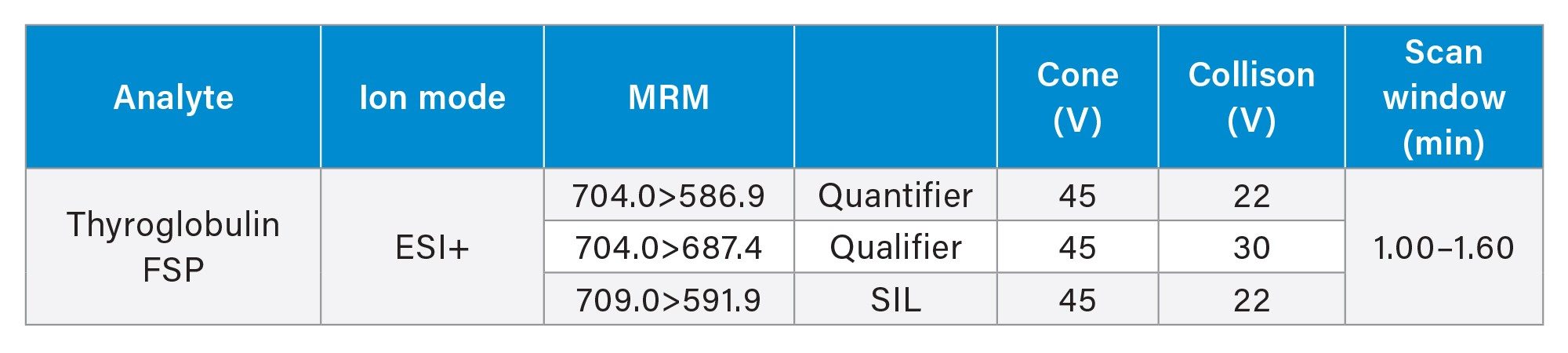
Method Events
Data Management
|
MS Software: |
MassLynx™ v4.2 Software (SCN 1042) |
|
Informatics: |
TargetLynx™ XS v4.2 Software |
Results and Discussion
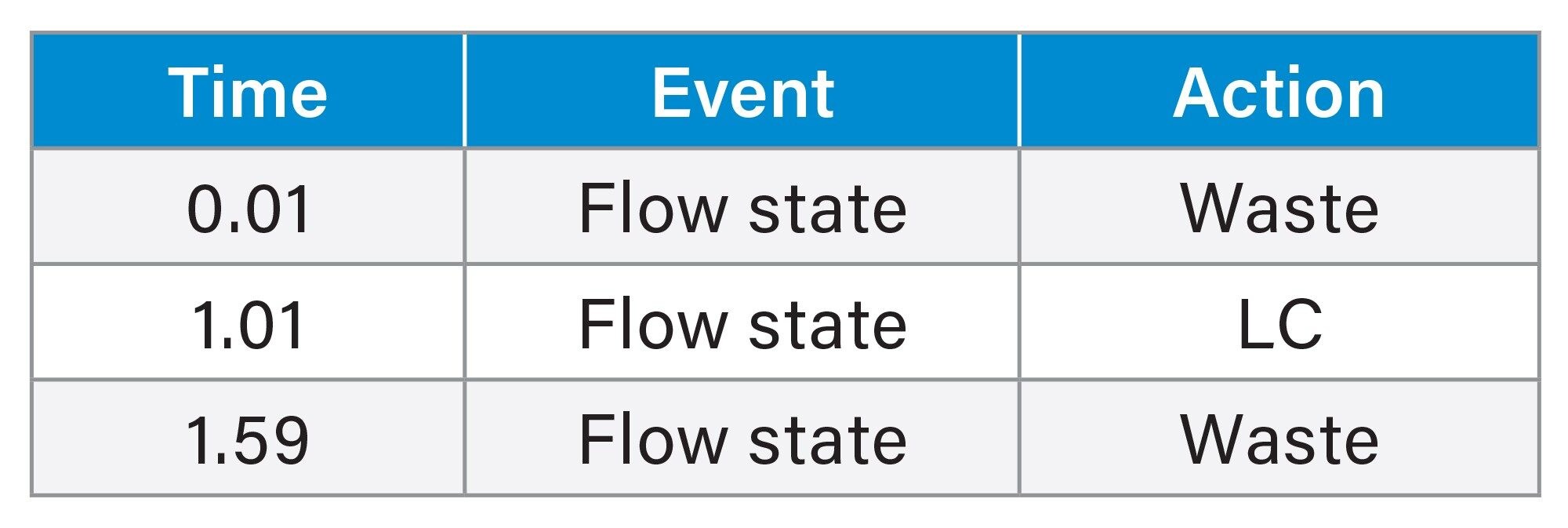
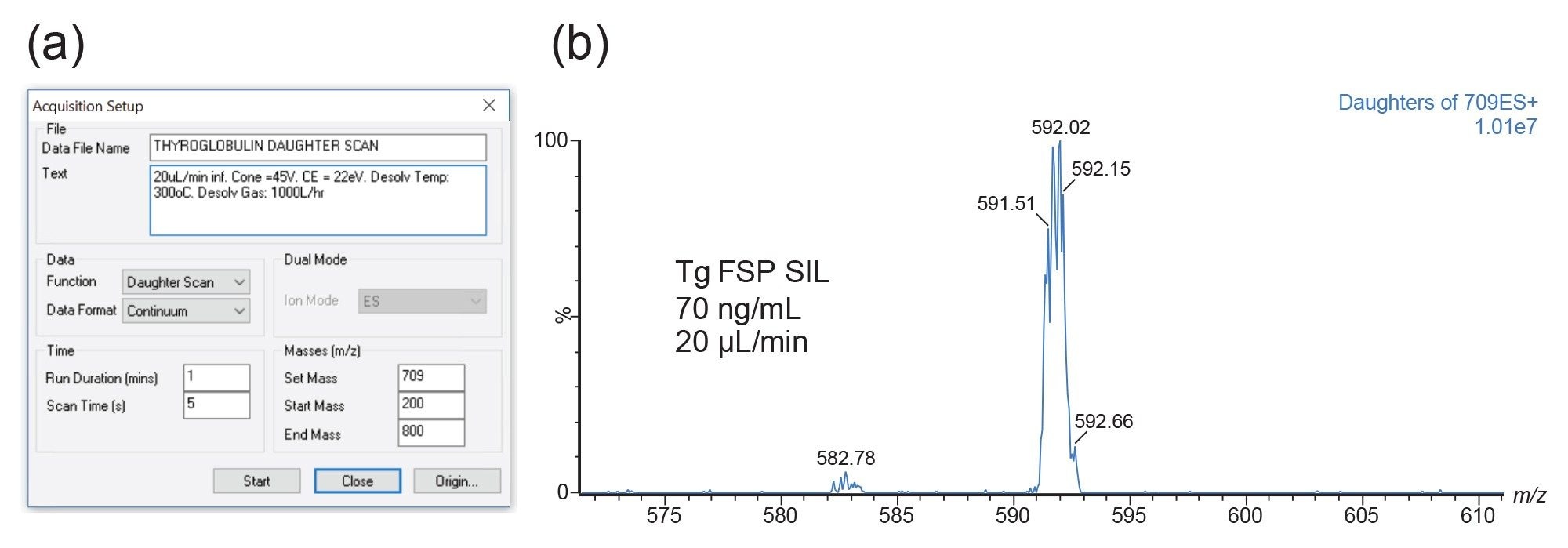
During the Xevo TQ Absolute Mass Spectrometer set-up, the performance was checked with an infusion of the FSP SIL solution prepared at 70 ng/mL in 2% acetonitrile, 0.5% formic acid. Mass calibration settings were confirmed, and daughter acquisitions were performed at 20 µL/min with scans performed every five seconds. An example of the daughter scan acquisition setup is shown in Figure 2a with the associated MS spectra in Figure 2b.
The XSelect Premier HSS T3 Column, 100 Å, 2.1 mm x 50 mm, 2.5 µm was used for the chromatographic retention of the Tg FSP peptide and its internal standard. The injection-to-injection cycle time is 3.3 minutes, allowing for injection of 18 samples an hour. Direct injection of the internal standard (0.05 ng/mL) was used for System Suitability Testing (SST) to help benchmark the system before analysis. A representative chromatogram is shown in Figure 3 alongside SST data across four analytical runs.
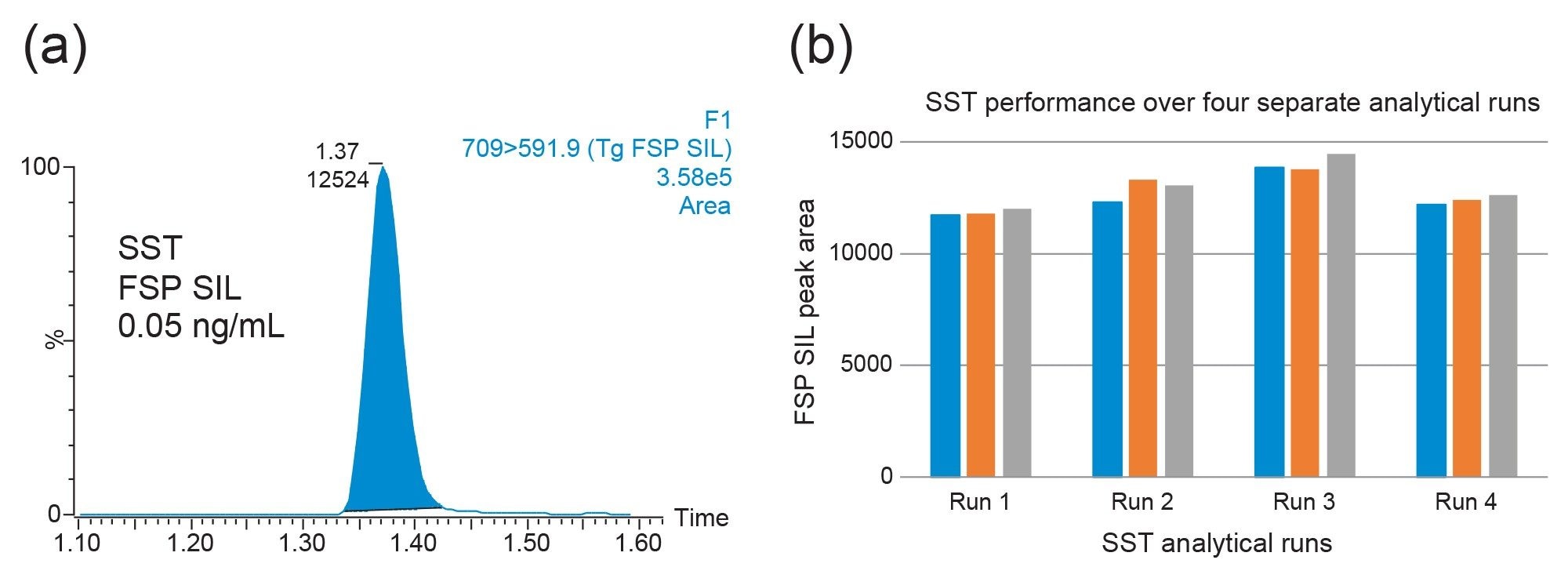
Following extraction of serum samples, the method was shown to be linear from 0.1 – 50 ng/mL, with r2 > 0.995 across all analytical runs. No significant carryover samples were observed from samples at 200 ng/mL, with peaks in subsequent blank samples <25% of the peak area at the 0.1 ng/mL concentration level. Dilution of samples prepared at both 100 ng/mL (1 in 5 dilution) and 500 ng/mL (1 in 25 dilution) with surrogate serum down to 20 ng/mL were acceptable, with accuracies of 97% and 99%, respectively.
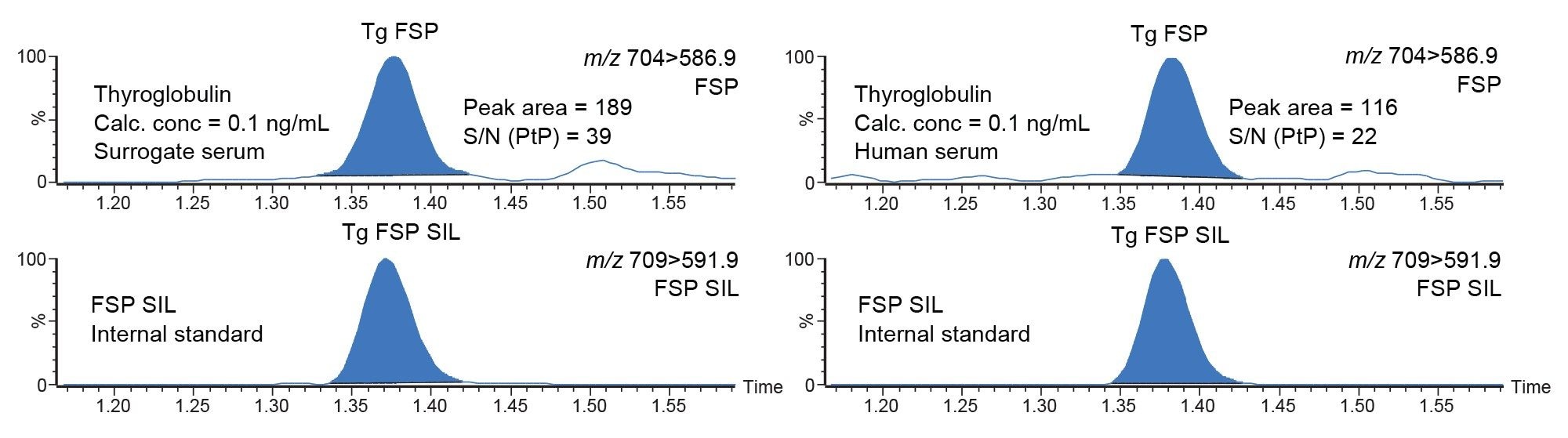
Analytical sensitivity investigations were performed across the lower end of the calibration range, at concentrations of 0.05, 0.08, 0.1, 0.2, and 1 ng/mL with ten replicates across three analytical runs (n=30). Investigations demonstrate that a concentration of 0.08 ng/mL can be measured reproducibly with <20% RSD. However, a consistent S/N value >10:1 can only be achieved at a concentration of 0.1 ng/mL. Representative chromatograms at 0.1 ng/mL in both surrogate serum and human serum are shown in Figure 4.
Matrix effects were evaluated by extracting six individual human serum samples in triplicate and post-spiking samples with a high concentration of FSP peptide and FSP SIL peptide. Endogenous thyroglobulin concentrations were compensated for through extraction of additional blank samples and then compared to FSP and SIL peptides prepared in injection solvent. Matrix Effects based on peak area ranged from 79% to 104%, with <9% RSD. Matrix effects based on analyte: internal standard response ratio ranged from 96% to 105%, with <4% RSD, demonstrating the internal standard compensates for changes in matrix effects observed for the method.
Interference testing was performed using a range of endogenous compounds (cholesterol, triglycerides, bilirubin, uric acid, albumin, and creatinine). No significant interference was observed with recoveries ranging from 90-103% at 0.3 ng/mL and 84-99% at 30 ng/mL serum Tg. Interference of TgAb was evaluated by a one in five dilution of Tg negative serum containing 786 U/mL TgAb with a 30 ng/mL serum sample. Recovery of Tg was 95% compared to a control sample.
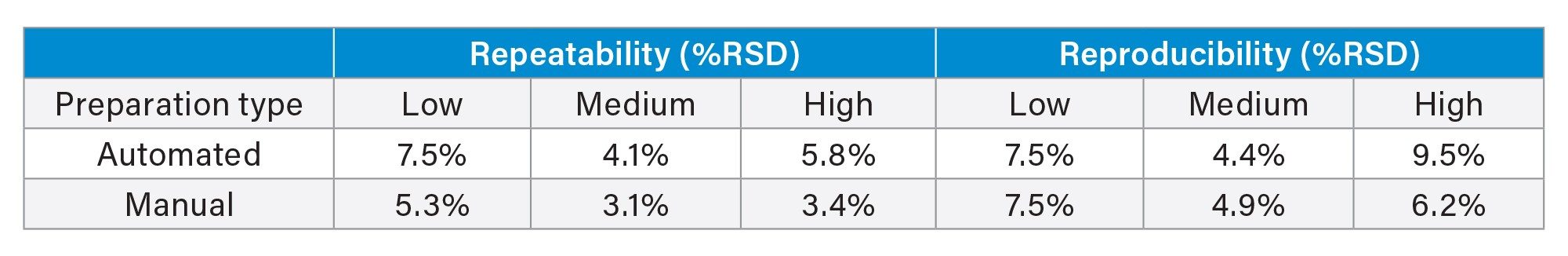
Manual and automated precision were evaluated for the method at low, medium, and high (0.3, 3, and 35 ng/mL) QC concentrations over five analytical runs, with five replicates at each concentration (n=25) (Table 1). Total reproducibility and repeatability of the automated precision assessments using the Andrew+ Pipetting Robot were ≤9.5% RSD. Total reproducibility and repeatability of the manual precision assessments were ≤7.5% RSD.
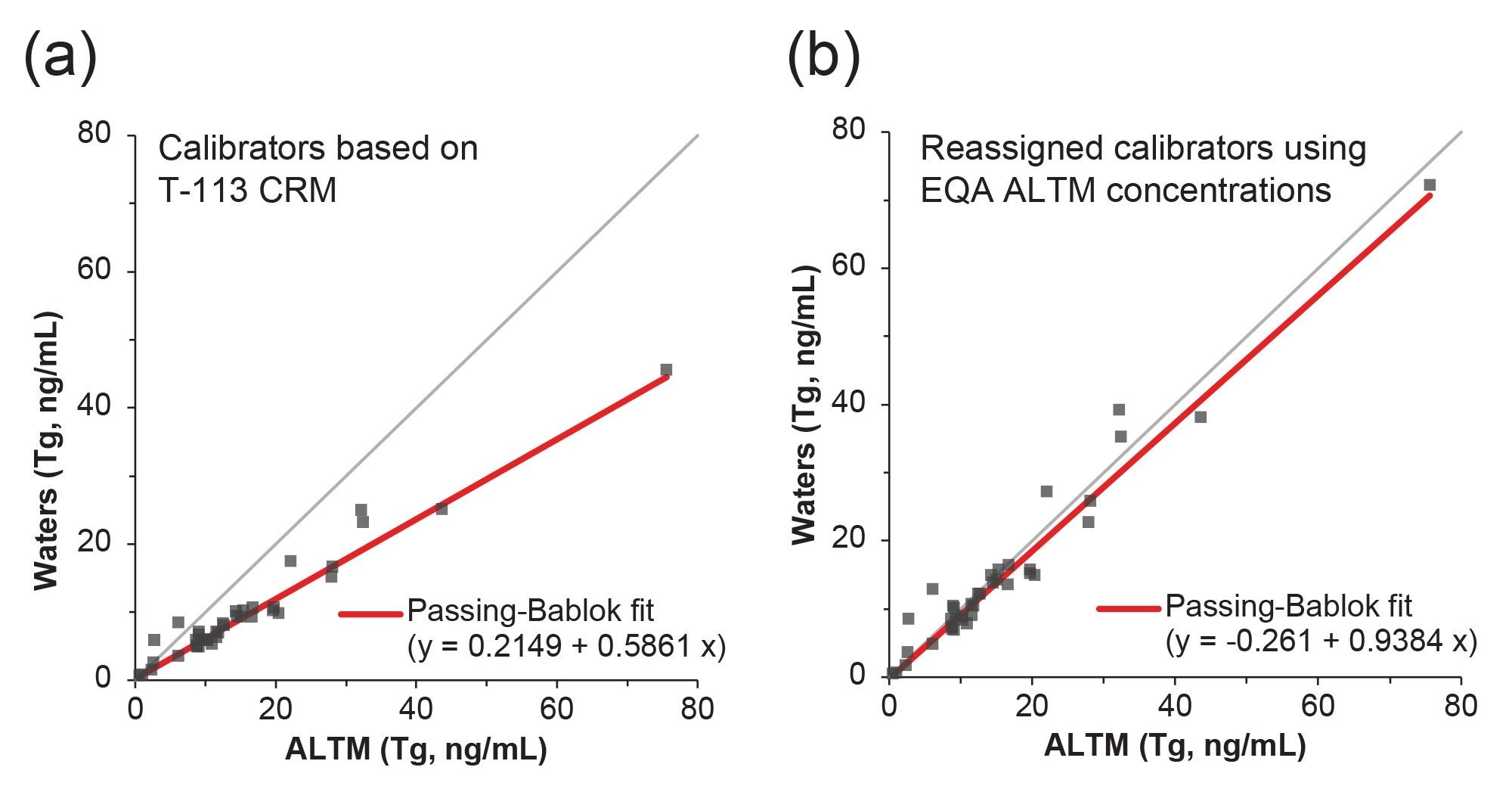
To evaluate method performance, 38 samples from UK NEQAS were evaluated and compared to the method All Laboratory Trimmed Mean (ALTM). Both a Passing-Bablok regression and Bland-Altman agreement were applied to the data set to assess the performance observed compared to the ALTM values, with the Passing-Bablok results shown in Figures 5a. The Passing-Bablok analysis demonstrates significant proportional bias with a regression of y=0.2149 + 0.5861x. This is further verified by the Bland-Altman agreement which demonstrates a -40.1% method bias of the developed method against the ALTM, indicating possible differences between method calibration. This was confirmed through reassignment of the in-house calibrators using the EQA ALTM, leading to a reduction in method bias to -5.5%, and a Passing-Bablok regression of y=-0.261 + 0.9384x (Figure 5b), indicating differences in the metrological traceability of the calibration material as previously observed.1,2
Conclusion
A LC-MS/MS clinical research method for serum thyroglobulin was developed using the SISCAPA Workflow, followed by analysis using ACQUITY UPLC I-Class PLUS FL System and the Xevo TQ Absolute Mass Spectrometer. The method provides analytical sensitivity down to 0.1 ng/mL from 250 µL serum, while providing sufficient sample for re-analysis.
The method demonstrates excellent linearity across the calibration range, with no significant carryover, interferences, and matrix effects. Total reproducibility and repeatability of the method was ≤9.5% RSD for manual and automated sample preparation, using the Andrew+ Pipetting Robot. In addition, the Andrew+ Pipetting Robot can minimize user touch-time, allowing a full plate to be prepared within four hours without user intervention.
References
- Netzel BC, Grant RP, Hoofnagle AN, Rockwood AL, Shuford CM, Grebe SK. First Steps toward Harmonization of LC-MS/MS Thyroglobulin Assays. Clin Chem. 2016 Jan;62(1):297-9. doi: 10.1373/clinchem.2015.245266. Epub 2015 Oct 1. PMID: 26430076; PMCID: PMC4794333.
- Shi J, Phipps WS, Owusu BY, Henderson CM, Laha TJ, Becker JO, Razavi M, Emrick MA, Hoofnagle AN. A distributable LC-MS/MS method for the measurement of serum thyroglobulin. J Mass Spectrom Adv Clin Lab. 2022 Sep 19;26:28-33. doi: 10.1016/j.jmsacl.2022.09.005. PMID: 36388059; PMCID: PMC9641599.
Acknowledgements
We would like to thank Ally Mathews and colleagues at the University Hospitals Birmingham NHS Foundation Trust for the provision of anonymized samples for method evaluation. In addition, we would like to thank Donna Austin and colleagues at Synnovis, Guys and St Thomas’ Hospital, London for calibrator comparisons during method development.
Waters, Andrew+, Xevo, ACQUITY, UPLC, XSelect, MassLynx, and TargetLynx are trademarks of Waters Technologies Corporation. SISCAPA is a trademark of Anderson Forschung Group LLC. cOmplete is a trademark of Roche Diagnostics GMBH. Axygen is a trademark of Axygen Inc. Trizma is a trademark of Merck KGAA. All other marks are the property of their respective owners.
720008167, December 2023