For research use only. Not for use in diagnostic procedures.
A flexible universal method was developed for the analysis of inter-class separations of neutral and amphipathetic lipids. Since the goal of the experiment is to achieve flexible parameters providing the separation of lipids by class, the core methodology using the ACQUITY UPC2 BEH column, methanol/acetonitrile modifier with ammonium formate as an additive provided the best results.
The analysis of complex lipids has historically been a challenging task and may require a variety of analytical techniques. Lipids are generally recognized as hydrophobic compounds, but the properties of complex lipids containing phosphorus, sulfur, sugar, and nitrogen have a wide polarity range. Developing separation-based methodology utilizing a single technique is recognized as a common challenge for scientists researching lipidomic and metabolomic applications.
Recent advances in technology have revived the exploration of supercritical fluid chromatography (SFC) as a viable analytical technique. Research and development focused on improvements of SFC instrumentation has provided a holistically designed chromatographic system that utilizes liquid CO2 as a mobile phase to leverage the chromatographic principles and selectivity of normal phase chromatography while providing the ease-of-use of reversed phase LC (RPLC). This new separation technique is referred to as UltraPerformance Convergence Chromatography (UPC2).
In this application note, UPC2 technology is implemented for the analysis of lipid class separation. Method variables influencing the peak integrity and chromatographic separation for a mixture of lipids with different degrees of polarity are explored. The experiments were designed to understand the chromatographic behavior of lipids in a controlled setting using a variety of lipid extracts. Acyl chain length and a number of double bond influences were investigated using single moiety standards. The methodology parameters were tested using lipid extracts composed of intra-class components. The method conditions are applied to biological lipid extracts, whereas method adjustments are investigated to manipulate the chromatography based on the goal of the analyst. Insights from these method variable manipulations help to scope the development of targeted lipid profiling and screening protocols.
|
CER |
Ceramides |
|
SM |
Sphingomyelin |
|
PG |
Phosphatidylglycerol |
|
PE |
Phosphatidylethanolamine |
|
PC |
Phosphatidylcholine |
|
LPC |
Lyso-Phosphatidylcholine |
|
LPE |
Lyso-Phosphatidylethanolamine |
Samples and standards were purchased from Avanti Polar Lipids. Mix 1 and Mix 2 were brain (porcine) extracts except LPC and PG which were egg (chicken) extracts. Stocks were prepared in 50:50 chloroform/methanol. Working lipid mixtures were prepared to the specified concentration.
|
Mix 1: |
Ceramide, SM, (0.05 mg/mL) PG, PE, PC, (0.1 mg/mL) |
|
Mix 2: |
LPC, LPE, (0.05 mg/mL) |
|
Mix 3: |
1:1 of [mix 1] and [mix 2] |
|
UPC2 conditions |
|
|---|---|
|
System: |
ACQUITY UPC2 |
|
Columns: |
ACQUITY UPC2 BEH and HSS C18 SB |
|
Column temp.: |
60 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
1 μL |
|
Flow rate: |
1.85 mL/min |
|
Back pressure: |
1500 psi |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
50:50 methanol/acetonitrile with 1 g/L ammonium formate |
|
Gradient: |
Refer to figures for detailed information |
|
Mass spectrometers: |
ACQUITY SQD and SYNAPT G2 MS |
|
Ionization mode: |
ESI positive |
|
Acquisition range : |
100 to 1500 Da |
|
Capillary voltage : |
3.5 kV |
|
Cone voltage : |
30 V |
|
Informatics: |
Empower 3 and MassLynx Software |
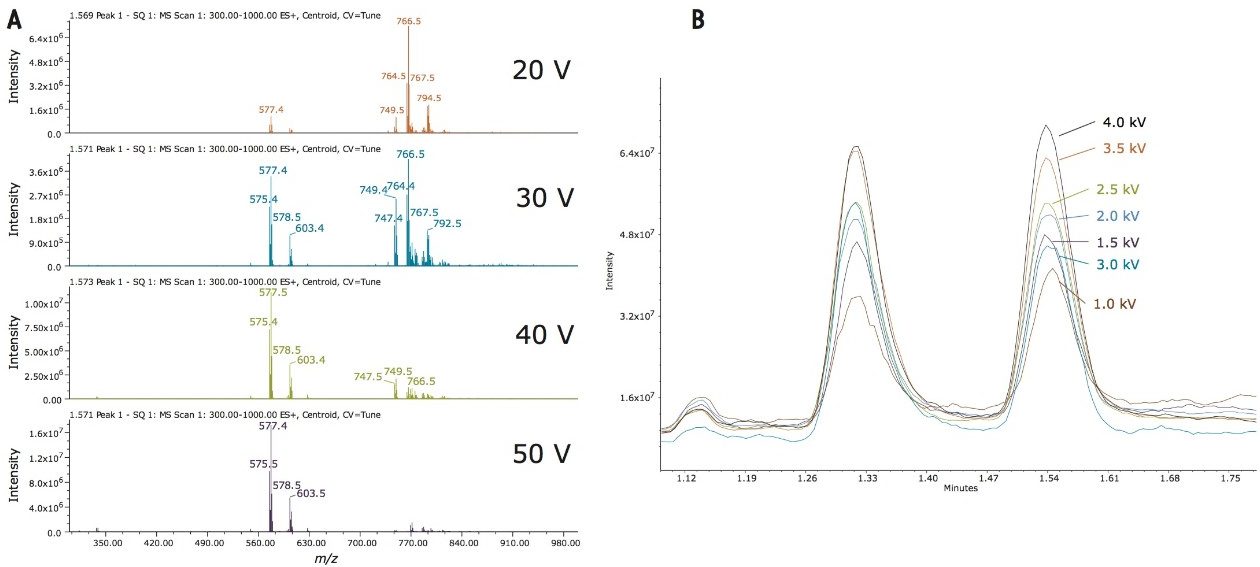
The ACQUITY UPC2 System was configured with an ACQUITY single quadrupole detector (SQD) for this preliminary investigation of chromatographic parameters. Injections of [Mix 3] were screened with different cone and capillary voltages to determine the best operating conditions. Based on the cone voltage screening results, 30 V was chosen for controlling overall in-source fragmentation of the optimal signal for the spectra of L-α-phosphatidylglycerol (egg PG) to provide precursor ion and valuable fragmentation information, as shown in Figure 1A. The optimal capillary voltage observed for all the peaks in the [Mix 3] was 3.5 to 4.0 kV, as shown in Figure 1B. The final MS conditions used 3.5 kV capillary voltage and 30 V cone voltage.
The sample preparation workflow was very convenient for use with the ACQUITY UPC2 System. The chloroform/methanol diluent provided good solubility without any noticeable adverse effects on peak shape. In a typical RPLC lipid analysis, the organic extract containing the lipids would have to be evaporated and re-constituted in a more compatible solvent. When using UPC2, however, the organic extract containing the lipids can be directly injected onto the system, thereby saving time and costs when analyzing hundreds of biological samples. Screening different column stationary phases typically changes selectivity during method development. Therefore, our initial approach was to screen three stationary phases including UPC2 CSH Fluoro-Phenyl, UPC2 BEH 2-EP, and UPC2 BEH. Optimal peak shape and selectivity was achieved on the UPC2 BEH stationary phase for the inter-class separation of the lipid mixture, as shown in Figure 2.
![Column screening of the [metdev] mixture of neutral and polar lipids.](/content/dam/waters/en/app-notes/2013/720004579/720004579en-f2.jpg.82.11-12-1268-704C.resize/img.jpg)
Method development screening columns and modifier indicated the bridged ethylene hybrid (BEH) silica columns provided the best selectivity and peak shape. The lipid classes were identified by MS ESI+ utilizing the parameters determined by the MS optimization experiments. Injections of the individual lipid mixtures verified current peak assignments. The method used for screening the columns was optimized to focus on the inter-class separation of analytes in [Mix 3]. The previous 12-minute method was reduced and completed in 5 minutes, as shown in Figure 3.
![Injection of [Mix 3] mixture using the ACQUITY UPC2 BEH column.](/content/dam/waters/en/app-notes/2013/720004579/720004579en-f3.jpg.82.7-15-1272-719C.resize/img.jpg)
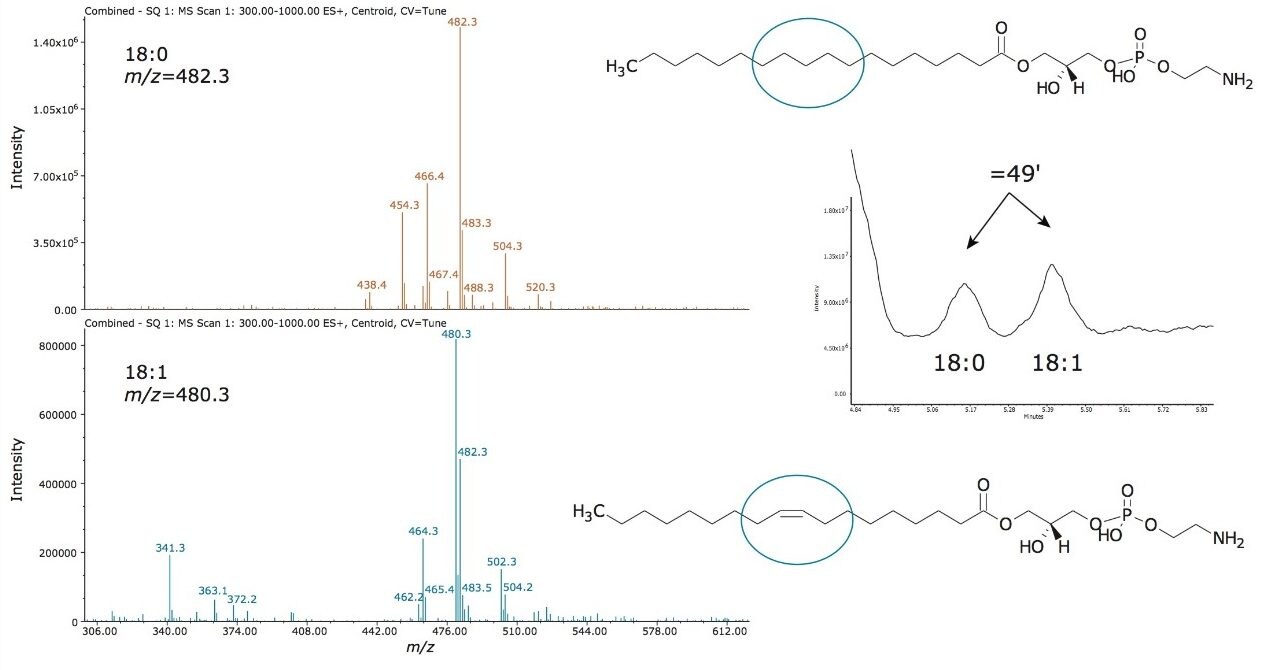
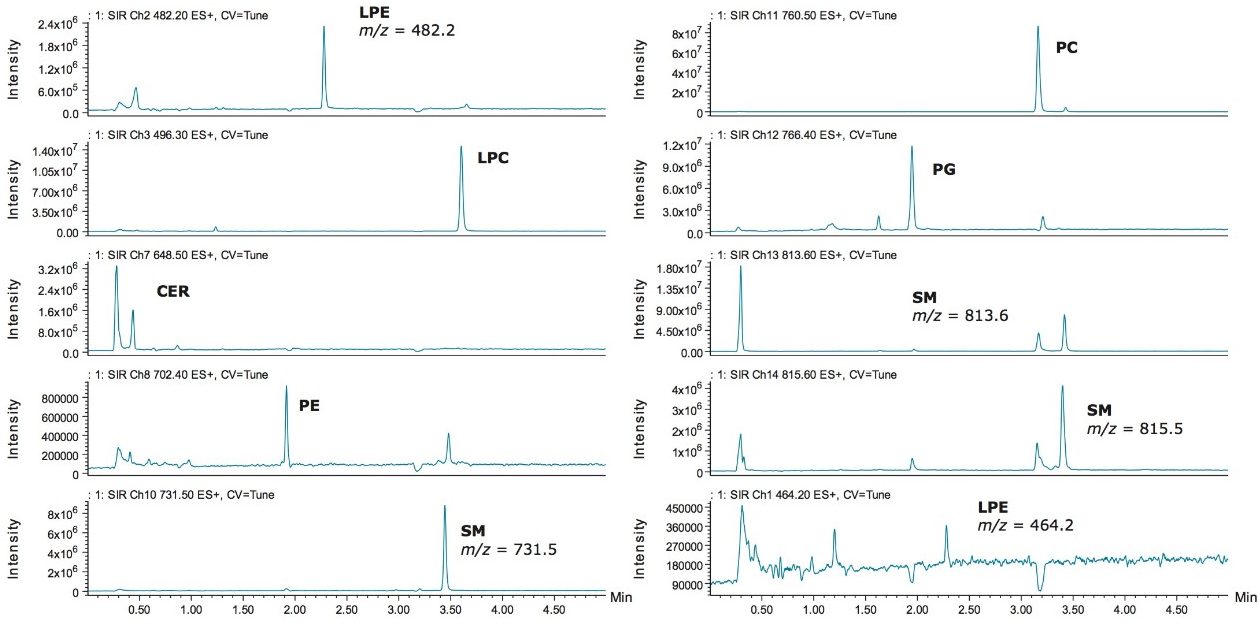
The lipid extracts purchased from Avanti provide documentation indicating various ratios of lipid intra-class constituents present within a respective standard extract. This was further confirmed when evaluating the mass spectral data for the two LPE peaks observed in chromatographic trace, as shown in Figure 4. It was found that the elution order of the molecular species within each lipid class depended on the number of double bonds on the acyl chain. Thus, the more saturated the acyl chain, the shorter the retention time. The acyl chain length has no effect on the elution order with each lipid class. Interestingly, the intra-class separation of LPE and SM standard extracts is observed when using the UPC2 BEH stationary phase. The other stationary phases that provided broader peaks may offer a better intra-class separation of the Avanti extract standards. This hypothesis will be explored in future studies.
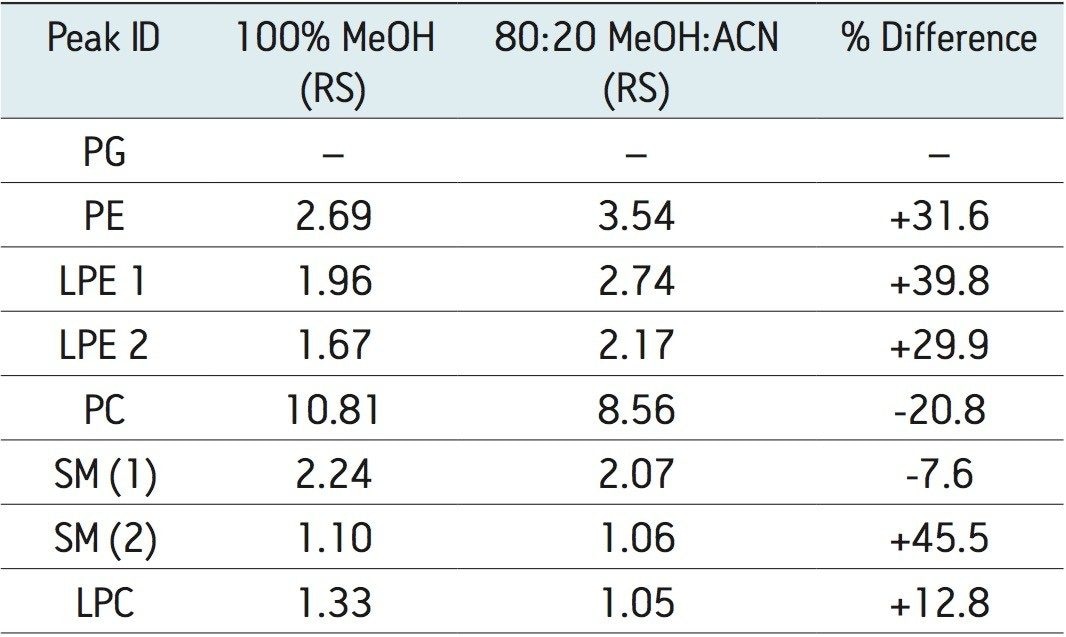
The method development process explored changes in gradient slope; however, changes in resolution were not significant. Experiments to manipulate retentivity were investigated. The variable which resulted in the greatest retentivity changes included the addition of a less polar solvent, such as acetonitrile, to the modifier. Two experiments were performed. The first was conducted with the original modifier composition of 100% methanol. The second experiment was conducted with modifier composition of 80% methanol and 20% acetonitrile. Both modifiers were doped with 1 g/L ammonium formate. In general, using acetonitrile in the modifier improved resolution, as shown in Table 1.
The methodology was used to investigate two biological samples, each for distinctly different purposes. The first example examines a mouse heart extract using the six-minute method for the rapid inter-class targeted screening of lipids with different polarity. The objective was to determine if the methodology could detect the presence of specific lipids within the hundreds of samples collected from the diabetic treatment study. This proof of concept approach will be used for further studies utilizing MS/MS quantification assessing increases of phospholipids and sphingolipids during treatment. As seen in Figure 5, many of the phospholipids and sphingolipids were easily identified in the mouse heart extract by using this targeted UPC2-MS method.
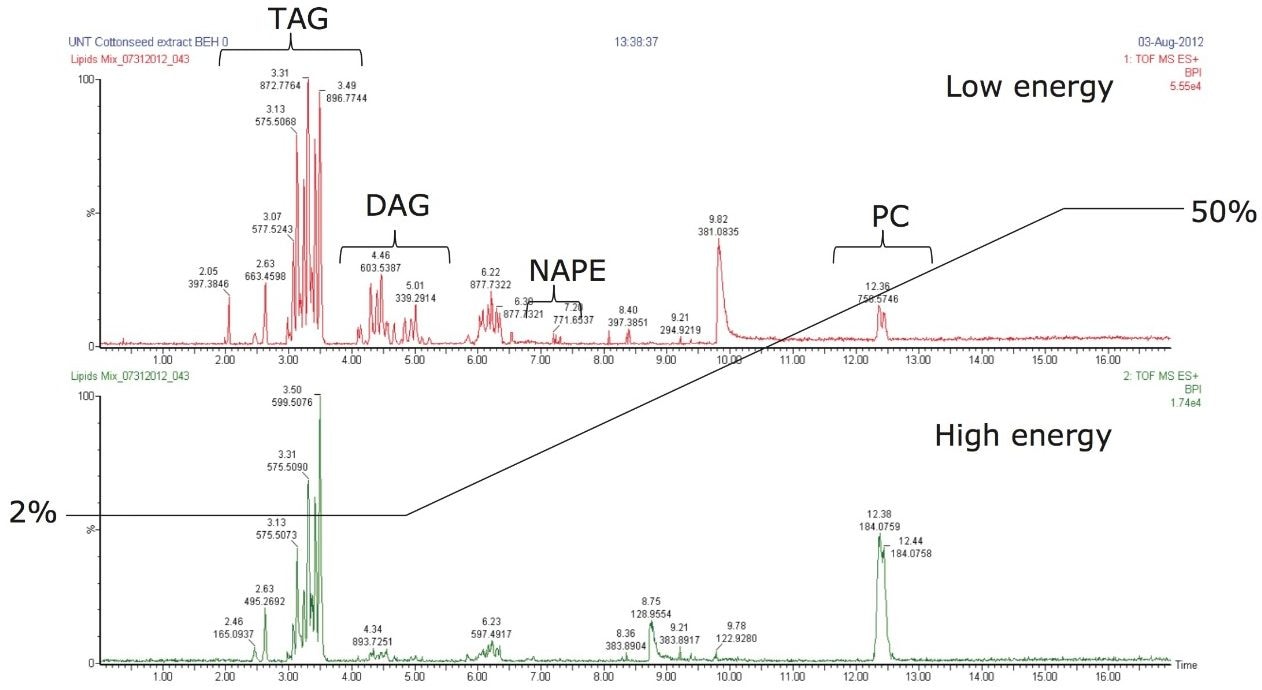
Neutral lipids, such as acylglyceride and cholesterol esters, are characteristically non-polar. Typically, plant metabolomic profiling distinguishes heterogeneous distribution of neutral lipids, such as triacylglycerols and diacylgylcerols2 at different conditions. The example in Figure 6 investigates lipids present in a cotton seed oil extract. The data was collected using MSE on a SYNAPT G2 Mass Spectrometer allowing for the characterization of the neutral lipids by precursor and product ion alignment. During the method development process, many neutral lipids eluted near the chromatographic void when starting the compositional gradient above 5% modifier using methanol (or methanol/acetonitrile). In this example, the UPC2-MS method was modified to retain the neutral lipids by reducing the starting percentage of modifier. The modifier was ramped to elute the polar lipids, known to be present as membrane lipid classes in cotton embryos.2 By using this approach, the chromatography can be altered to provide greater retention, and often greater specificity for the neutral lipids. From an analytical technique perspective, the elution mechanisms are conceptually similar to performing a mobile phase gradient elution profile by liquid chromatography.
A flexible universal method was developed for the analysis of inter-class separations of neutral and amphipathetic lipids. Since the goal of the experiment was to achieve flexible parameters providing the separation of lipids by class, the core methodology using the ACQUITY UPC2 BEH column, methanol/acetonitrile modifier with ammonium formate as an additive provided the best results. The gradient methodology and run time was adjustable to focus on rapid screening or comprehensive profiling of lipids in biological samples. The lipid sample preparation workflow is suitable for UPC2. The organic phase of the biological lipid extract can be directly injected onto the ACQUITY UPC2 System with MS detection, thereby saving time and operating costs. The rapid inter-class screening provided an analysis within seven minutes including re-equilibration. The MS spectral information confirmed instances of intra-class separation by distinguishing between the degrees of saturation, as demonstrated for the Lyso- Phosphatidylethanolamine separation on the ACQUITY UPC2 BEH column. Resolution can be increased within the chromatographic space by the addition of acetonitrile. For this methodology, an increase of 30% to 40% resolution can be observed between lipid classes for the majority of the analytes. The method development knowledge gained from these experiments build a foundation for the applicability of lipid analysis by convergence chromatography.
720004579, February 2013