This application note presents the comprehensive comparison of both tandem quadrupole and time-of-flight mass spectrometers for the quantitative MRM-based analysis of peptide biomarkers.
The microfluidic LC platform combined with the Xevo TQ-S tandem quadrupole mass spectrometer is the ideal LC-MS system for translational research studies where low level quantitation of tryptic peptides is required.
Targeted LC-MS-based methodologies are increasingly applied in the post-discovery proteomics area with an emphasis on validation, which is the first of many phases in translational research.1 These types of experiments are technologically challenging, because they require the analysis of large sample cohorts with high throughput, high sensitivity, large linear dynamic range, and excellent selectivity. Multiple Reaction Monitoring- (MRM) based methods have the potential to provide the performance required to improve biomarker acceptance.
This application note presents the comprehensive comparison of both tandem quadrupole and time-of-flight mass spectrometers combined with nanoscale and microfluidics (ionKey) liquid chromatography platforms for the quantitative MRM-based analysis of peptides in a tryptically-digested, non-fractionated, undepleted human serum sample. The LC and MS platforms were combined to form eight distinct LC-MS configurations. Moreover, these platforms were compared in terms of throughput, sensitivity, linearity, and reproducibility in order to demonstrate their suitability for the analysis of large sample cohorts in translational research studies.
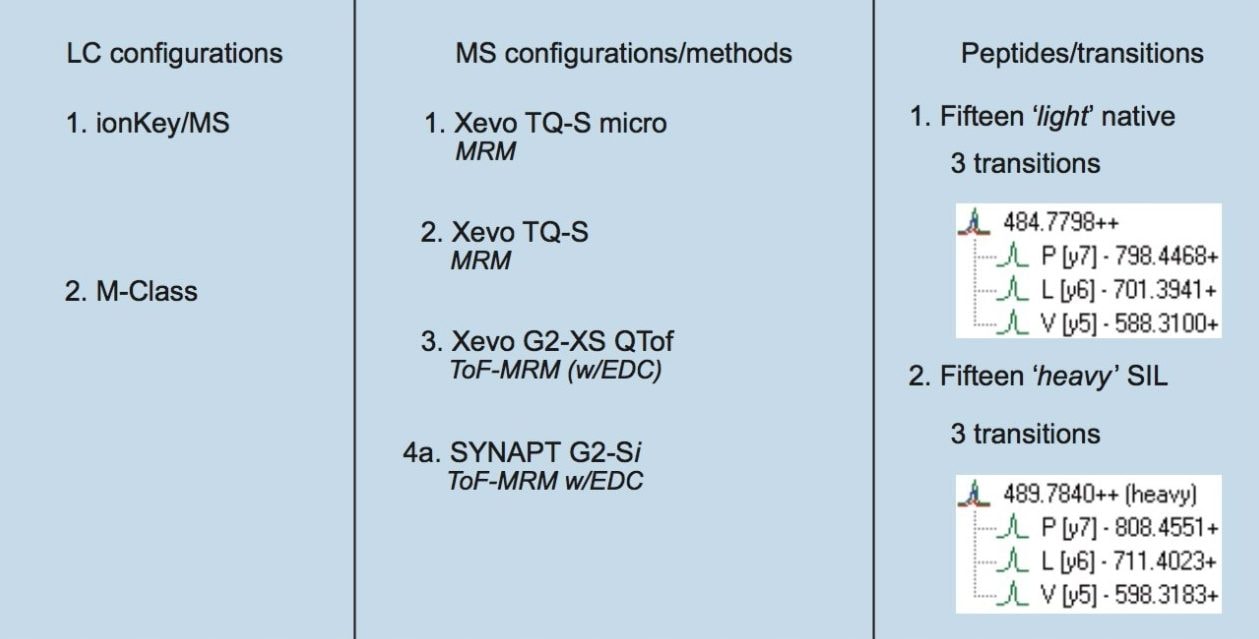
Fifteen stable isotope-labeled (SIL) peptides – representing putative cardiovascular disease protein biomarkers – were obtained from PepScan (Lelystad, Netherlands). The peptides were simultaneously spiked as a dilution series from 6.25 amol to 12.5 fmol/µL in a non-fractionated, undepleted, tryptically-digested human serum sample.
20 µL of non-fractionated, undepleted human serum was diluted with 80 µL of 50 mM ammonium bicarbonate(aq), and denatured in the presence of 10 µL of 1% RapiGest detergent solution at 80 °C for 45 minutes. The plasma proteins were reduced in the presence of 5 µL of 100 mM dithiothreitol at 60 °C for 30 minutes, and alkylated in the dark in the presence of 6 µL of 200 mM iodoacetamide at ambient temperature for 30 minutes. Proteolytic digestion was initiated by adding 40 µL of 1 µg/µL sequencing grade, modified trypsin, and then incubated overnight at 37 °C. Breakdown of the acid-labile detergent was achieved in the presence of 1% TFA at 37 °C for 45 minutes. The peptide solutions were centrifuged at 13,000 rpm for 10 minutes, and the supernatants collected. The resulting solution was diluted with water prior to use in order to give a serum digest on-column amount of 200 ng (assuming a total protein concentration of 100 g/L).
|
LC system: |
ACQUITY UPLC M-Class |
|
Trap column: |
ACQUITY UPLC M-Class Symmetry C18, 5 μm, 180 μm x 20 mm |
|
Analytical column: |
ACQUITY UPLC M-Class HSS T3 C18, 1.8 μm, 75 μm x 250 mm |
|
Column temp.: |
35 °C for analytical column |
|
Sample temp.: |
8 °C |
|
Injection volume: |
1 μL |
|
Flow rate: |
300 nL/min |
|
Mobile phase A: |
0.1% formic acid |
|
Mobile phase B: |
Acetonitrile containing 0.1% formic acid |
|
Gradient: |
3–40% mobile phase B over 90 min |
1 µL of sample was transferred with 0.1% formic acid(aq) to the trap column at a flow rate of 5 µL/min for 3 minutes. Mobile phase A was 0.1% formic acid(aq) and mobile phase B was acetonitrile containing 0.1% formic acid. After desalting and pre-concentration, the peptides were eluted from the trap column onto the analytical column, and separated with a gradient of 3 to 40% mobile phase B over 90 minutes at a flow rate of 300 nL/min, followed by a 2 minute column wash at 85% mobile phase B. The columns were then re-equilibrated to initial conditions for 20 minutes. The analytical column temperature was maintained at 35 °C.
|
LC system: |
MRM using Xevo TQ-S, Xevo TQ-S micro, Xevo G2-XS QTof, and SYNAPT G2-Si |
|
Device: |
iKey Peptide BEH C18, 1.7 μm, 150 μm x 100 mm |
|
Column temp.: |
35 °C |
|
Sample temp.: |
8 °C |
|
Injection volume: |
1 μL |
|
Flow rate: |
1 μL/min |
|
Mobile phase A: |
0.1% formic acid |
|
Mobile phase B: |
Acetonitrile containing 0.1% formic acid |
|
Gradient: |
3–40% mobile phase B over 45 min |
1 µL of sample was loaded directly onto the iKey Separation Device. Peptides were separated with a gradient of 3 to 40% mobile phase B over 45 minutes at a flow rate of 1 µL/min followed by a 6 minute column wash with 85% mobile phase B. The ionKey was then re-equilibrated to initial conditions for 9 minutes. The analytical column temperature was maintained at 35 °C.
|
MS system: |
MRM using Xevo TQ-S, Xevo TQ-S micro, Xevo G2-XS QTof, and SYNAPT G2-Si |
|
Ionization mode: |
ESI+ |
|
Ion source temp.: |
100 °C |
|
Capillary voltage: |
3.4 kV |
|
Cone voltage: |
30 V |
|
Cone gas flow: |
35 L/h |
|
Nanoflow gas pressure: |
0.2 bar |
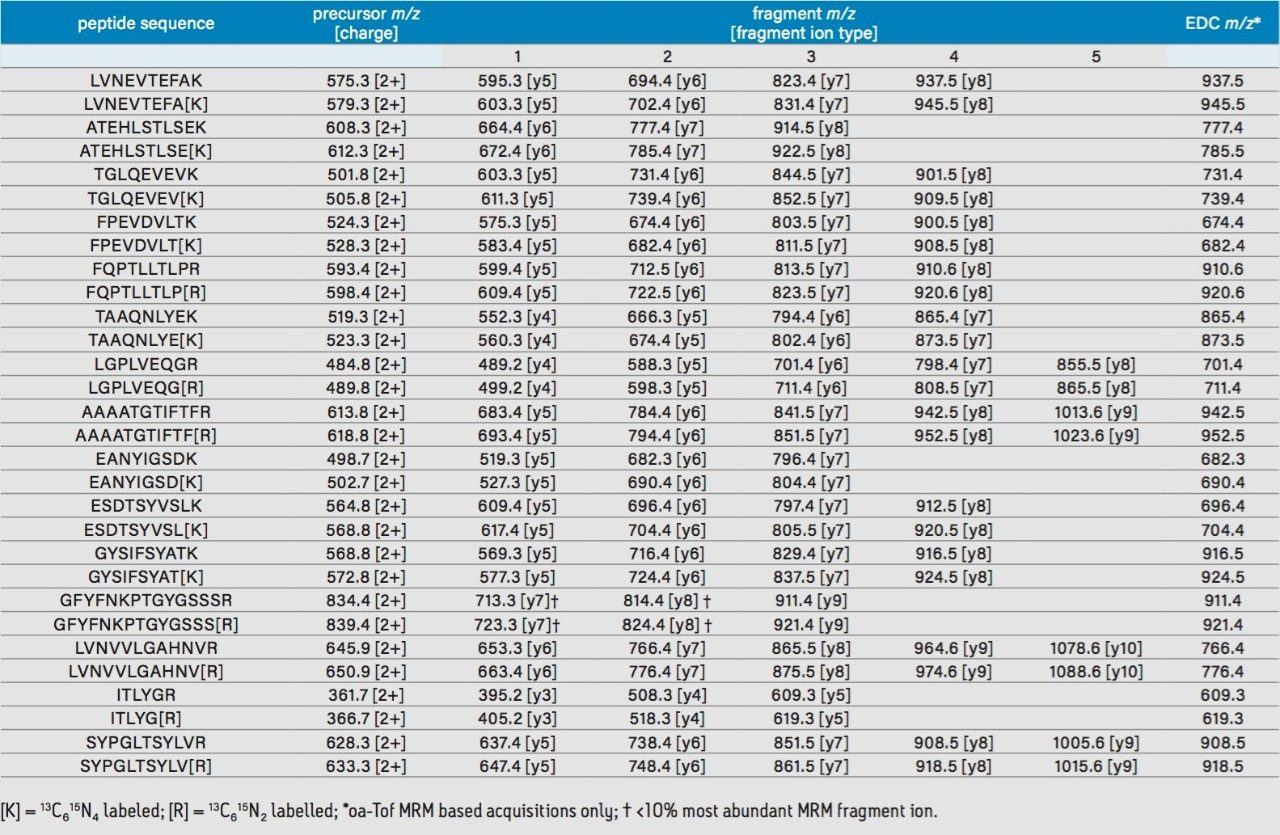
MRM analyses were performed using two tandem quadrupole mass spectrometers (Xevo TQ-S and Xevo TQ-S micro), and two hybrid quadrupole orthogonal acceleration time-of-flight (oa-Tof) mass spectrometers (Xevo G2-XS QTof and SYNAPT G2-Si). Endogenous and labeled peptides were targeted by at least three MRM transitions with a minimum of 10 data points over a chromatographic peak. Tandem quadrupole dwell and interscan delay times were automatically calculated by the operating software based on a minimum number of data points specified at half height across a chromatographic peak. Collision energies were set at fixed values for the tandem quadrupole instruments and ramped for the time-of-flight instruments. In addition, for the time-of-flight-based MRM acquisitions, integration and interscan delay times were manually set. Collision energies were ramped and initially calculated using the following regression equation, (0.034 x m/z) + 3.314 eV, and further optimized by CID fragmentation evaluation obtained by repeat injections of SIL peptides in the absence of matrix.
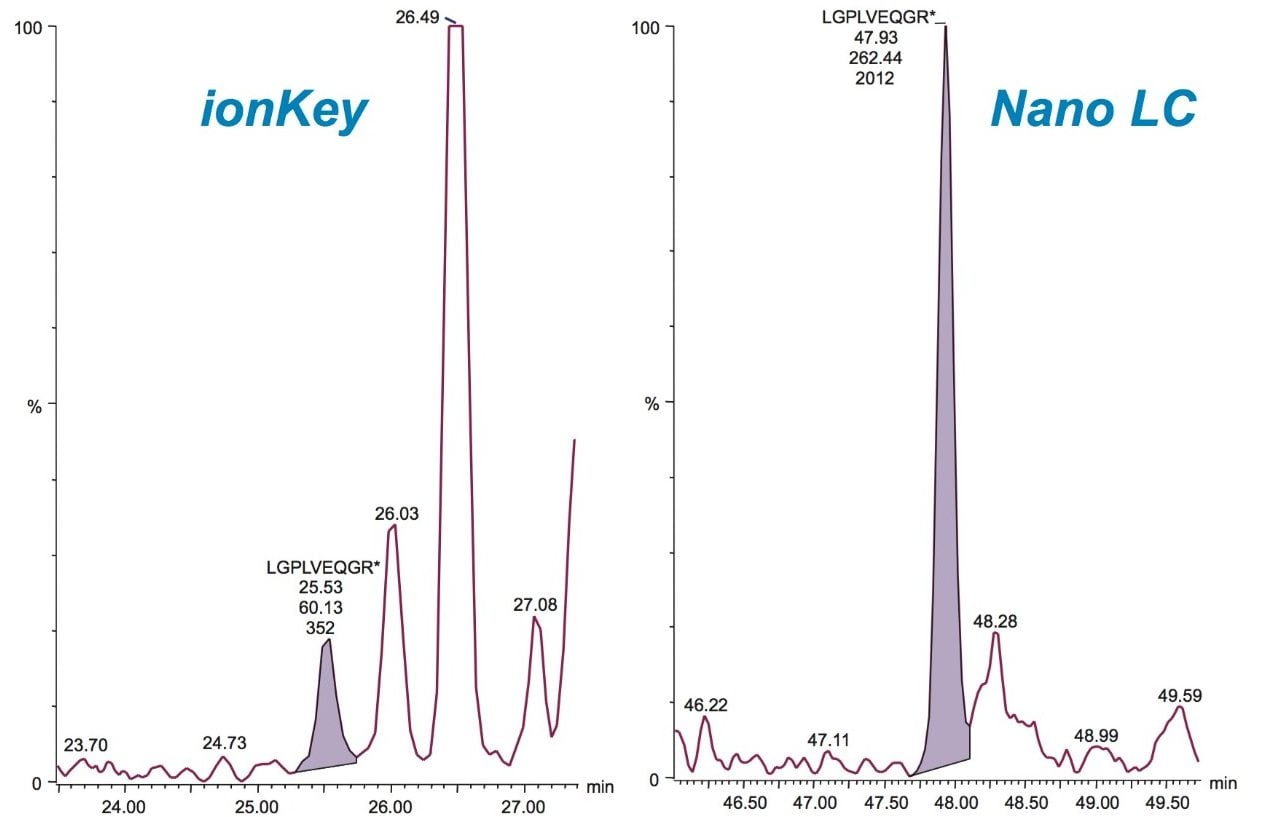
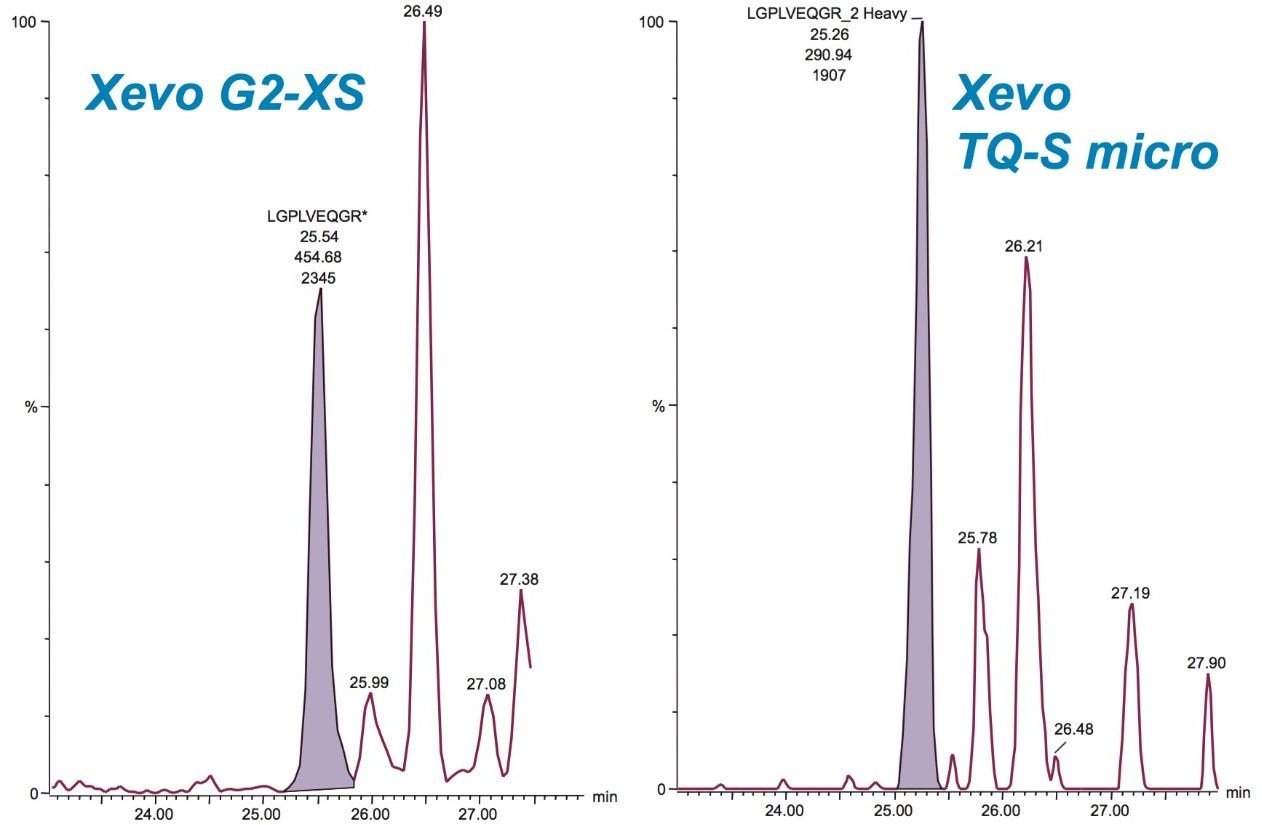
The fastest gradient separations possible were employed without introducing isobaric interferences for the fifteen SIL peptides monitored for both LC platforms. The microfluidics interface operates at a higher flow rate with a reduced number of connections compared to the nanoscale LC setup. Because of this, system volumes are more rapidly cleared, achieving faster gradient delivery and column conditioning. Consequently, extra column volumes – which normally can lead to band broadening – will be less critical. The complete experimental injection-to-injection cycle times were 1 and 2 hours for the microfluidics and nanoscale LC-based separations, respectively – providing a 2-fold increase in throughput for the microfluidics experiments. Figure 2 demonstrates this increase in throughput for one of the SIL peptides. Peak capacities under these gradient conditions were found to be similar for both LC configurations (data not shown)
The peak-to-peak, signal-to-noise ratio values measured at the 12.5 amol level for the nanoscale LC-MS platforms, and 125 amol level for the microfluidics LC-MS platforms, were found to be comparable on average across the various SIL peptides on all four MS platforms. Figure 3 demonstrates the similar sensitivity observed when using the microfluidic LC platform along with tandem quadrupole and time-of-flight mass MS platforms. Peak-to-peak, signal-to-noise values ranged from 2 to 30 for the nanoscale LC-MS platforms, and 2 to 100 for the microfluidics LC-MS platforms at the two levels described above. The median and mean lower limit of detection (LLOD) values across all MS platforms were 5 and 8 amol for the nanoscale LC-MS platforms, and 20 and 39 amol for the microfluidics LC-MS platforms, demonstrating nanoscale LC to be approximately 4 times the sensitivity of microfluidics LC. Figure 5 shows the average sensitivity across all SIL peptides for the four nanoscale LC-MS platforms in terms of both signal-to-noise ratio and limit of detection.
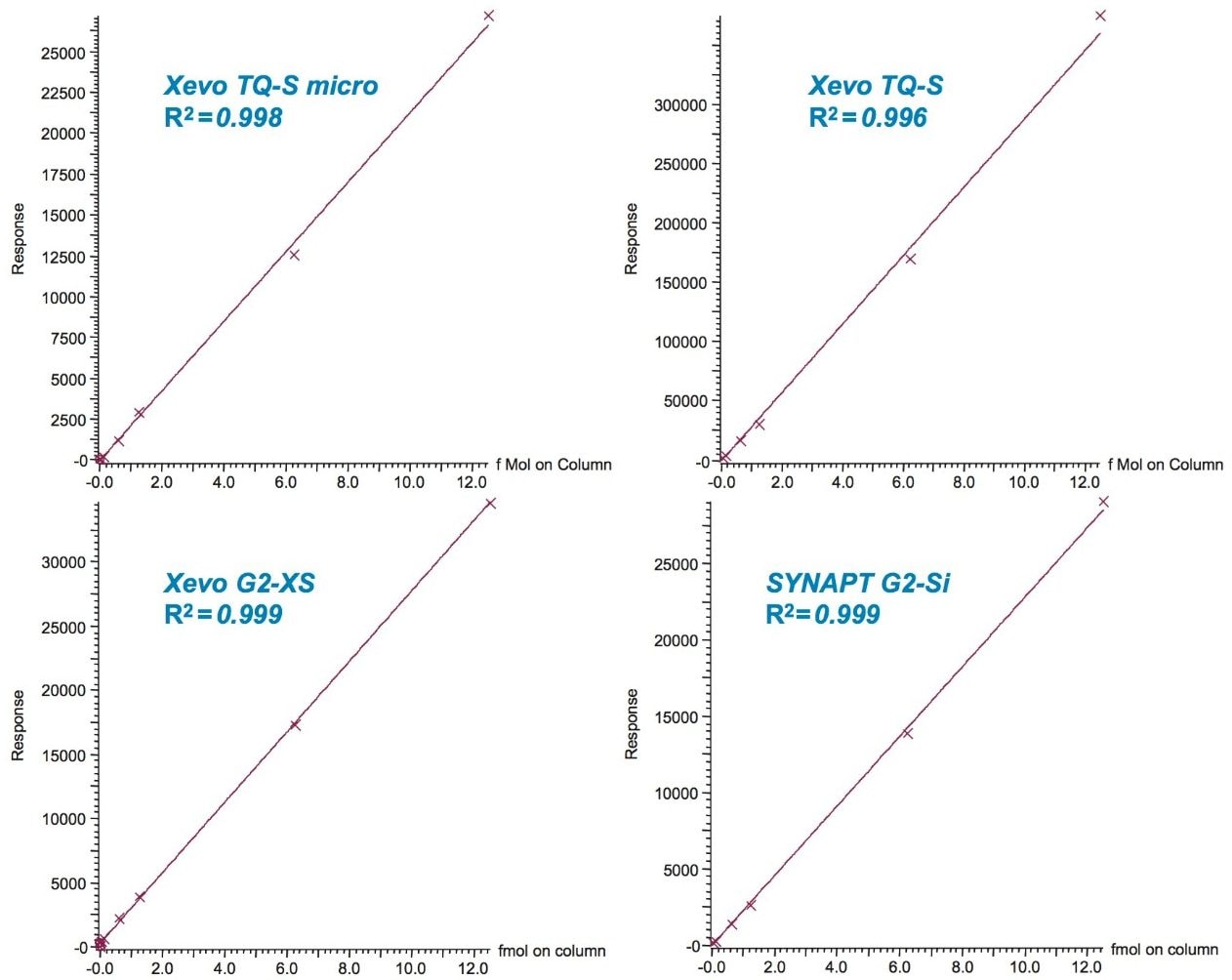
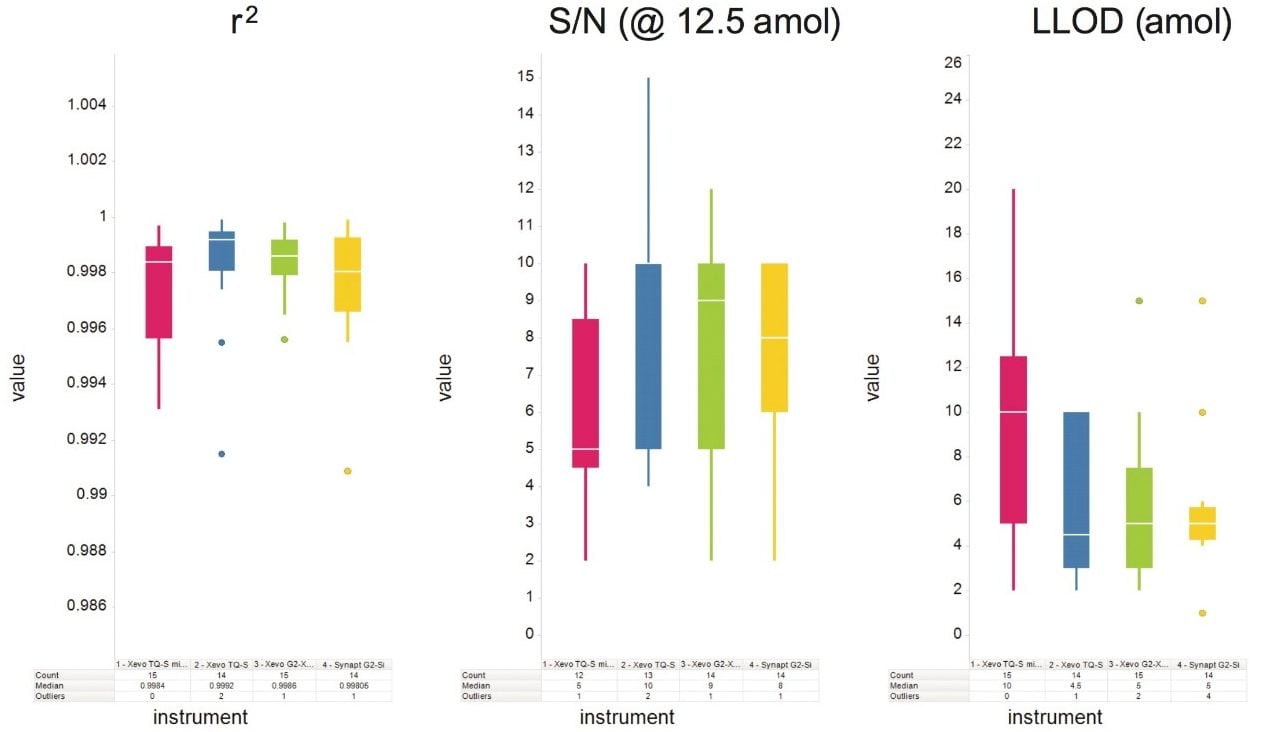
All eight LC-MS platforms demonstrated 1/x weighted linear behavior with r2 regression correlation coefficient values of 0.986 or greater for all SIL peptides over the tested ranges. Figure 4 demonstrates the linearity of all four MS platforms for one of the SIL peptides using the microfluidics LC platform. Figure 5 graphically summarizes the average r2 regression correlation coefficient values observed across all SIL peptides on all four nanoscale LC-MS platforms, as well as the S/N at 12.5 amol injected on-column and the estimated LLOD.
As the fifteen SIL peptides were spiked in a non-fractionated, undepleted, tryptically-digested human serum matrix, the quantitation of the endogenous peptides present in the matrix, within the measured linear dynamic range, was possible.
The average concentration and relative standard deviation (%RSD) were calculated for each of the peptides that were present within this range across all eight LC-MS platforms. Figure 7 illustrates the overall average sensitivity/reproducibility relationship for the eight LC-MS configurations studied, showing the earlier mentioned 4-fold sensitivity difference between LC platforms and improved reproducibility for the tandem quadruple mass spectrometers. The results plotted in Figure 6 show the individually-determined average concentrations as well as the average relative standard deviation (%RSD) for each LC-MS configuration for these peptides.
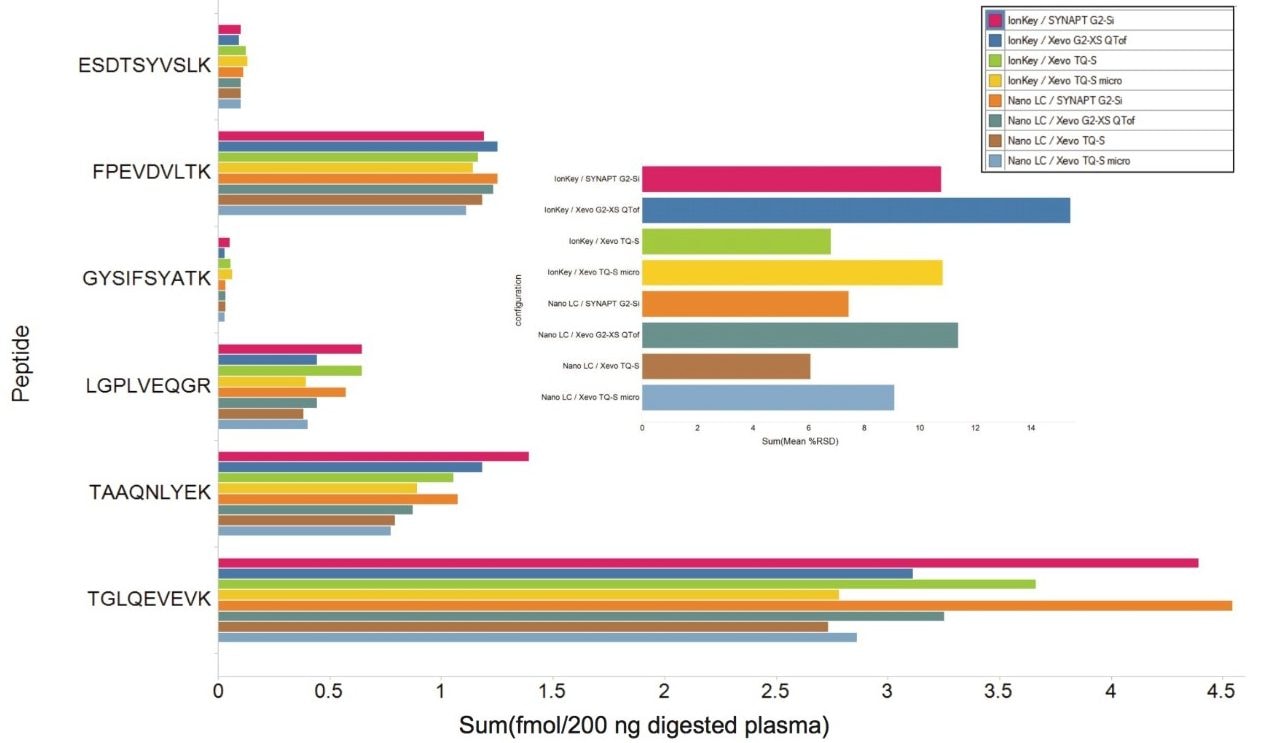
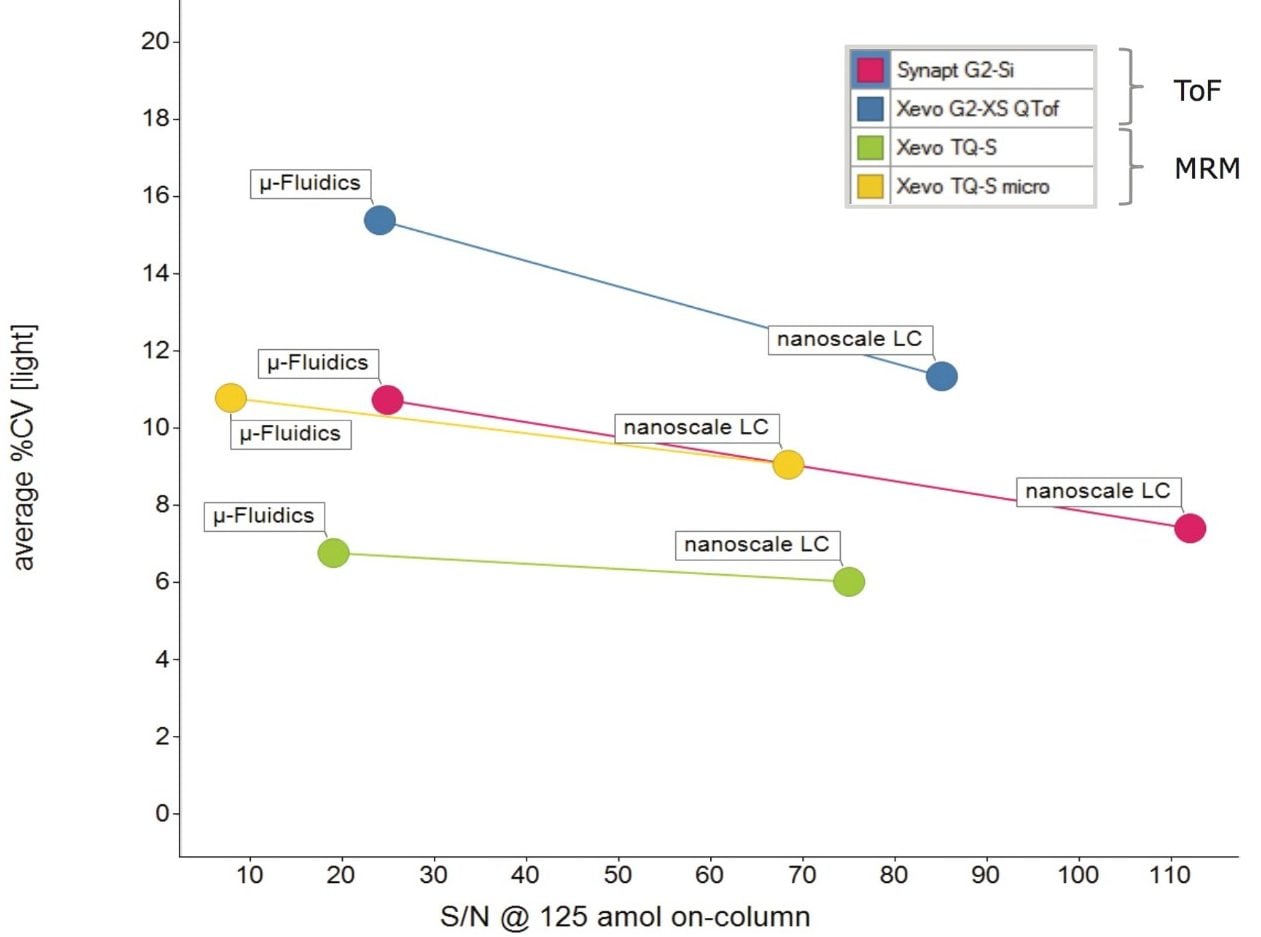
Throughput is increased when using the microfluidic LC-MS platforms when compared to nanoscale LC-MS platforms. However, this must be balanced against the increased sensitivity of the nanoscale LC-MS platforms. The limits of detection possible using the microfluidic LC-MS platforms were within the range of 3 to 250 amol, depending on the peptide and LC-MS platform used. The linearity of all eight LC-MS platforms were shown to be excellent across the ranges measured. In terms of reproducibility, all eight LC-MS platforms demonstrated %RSD values below 16%, with the tandem quadrupole LC-MS platforms showing increased reproducibility when compared to the high-resolution time-of flight platforms, especially the Xevo TQ-S platform.
When all of these factors are combined and considered, the increased throughput, usability, and reproducibility – balanced against a relatively small drop in sensitivity – make the microfluidic LC platform combined with the Xevo TQ-S tandem quadrupole mass spectrometer the ideal LC-MS system for translational research studies where low level quantitation of tryptic peptides is required.
720005719, July 2016