
Detection, characterization, and quantitation of the active ingredient/s and all other components in agrochemical formulations including impurities and degradation products are necessary to support product development, quality control, and product registration. Liquid chromatography (LC) techniques with photo diode array (PDA) detection have been used for the routine analysis of formulation samples. The addition of a mass detector in conjunction with UV detection can increase the specificity and selectivity of methods used during analytical testing to provide additional information about a sample in a single analysis.
In this application note, we present the analysis of a commercially available pesticide formulation which contained two active ingredients (AI): an insecticide AI 1, and tebuconazole, a triazole fungicide, AI 2. The triazole fungicides are a commonly used group of pesticides due to their potent activity against a broad spectrum of crop diseases.
The analysis of the formulation employed UV and mass detection and a dual-flow path liquid chromatography system capable of emulating HPLC or UHPLC separations. The ACQUITY Arc System enables existing HPLC methods to be performed, while also allowing the choice of transitioning to a UHPLC method that employs sub-3 μm particles for higher efficiency chromatographic separations.
Empower 3 Software was used for data acquisition and analysis, generating results which were used to flag impurities greater than specified %Area levels. The combined detection capabilities and data analysis provided the initial structural characterization of the unknown components.
Crop protection products provide solutions that decrease crop damage resulting in a food supply that is plentiful and of high quality.1 In the agricultural chemicals industry the analytical quality control of pesticide products is very important to ensure that a consistent and effective product reaches the customer.2 Detection, characterization, and quantitation of the active ingredient/s and all other components in the formulation including impurities and degradation products are necessary to support product development, quality control, and product registration. Liquid chromatography (LC) techniques with photo diode array (PDA) detection have been used for the routine analysis of formulation samples.2-4 The addition of a mass detector in conjunction with UV detection can increase the specificity and selectivity of methods used during analytical testing to provide additional information about a sample in a single analysis.
In this application note, we present the analysis of a commercially available pesticide formulation which contained two active ingredients (AI): an insecticide AI 1, and tebuconazole, a triazole fungicide, AI 2 (Figure 1). The triazole fungicides are a commonly used group of pesticides due to their potent activity against a broad spectrum of crop diseases.5 The analysis of the formulation employed UV and mass detection and a dual-flow path liquid chromatography system capable of emulating HPLC or UHPLC separations.6 The ACQUITY Arc System enables existing HPLC methods to be performed, while also allowing the choice of transitioning to a UHPLC method that employs sub-3 µm particles for higher efficiency chromatographic separations.
Empower 3 Software was used for data acquisition and analysis, generating results which were used to flag impurities greater than specified %Area levels. Empower’s Custom Fields allowed extra information to be derived from the results as custom calculations which were reported using tailored methods. The combined detection capabilities and data analysis provided the initial structural characterization of the unknown components.
All separations were performed on a Waters. ACQUITY Arc System equipped with a 2998 Photodiode Array (PDA) and an ACQUITY QDa Detector. Empower 3 Software was used for data acquisition and processing.
|
LC system: |
ACQUITY Arc |
|
Separation mode: |
Gradient |
|
Column: |
CORTECS C18+ 3.0 x 100 mm, 2.7 μm |
|
Solvent A: |
Water with 0.1% formic acid |
|
Solvent B: |
Acetonitrile |
|
Flow rate: |
0.80 mL/min |
|
UV detector: |
2998 Photodiode Array (PDA) |
|
PDA detection: |
210 to 400 nm |
|
Column temp.: |
50 °C |
|
Injection volume: |
5 μL |
|
Gradient conditions: |
0 min 20% B, 10 min 80% B, 11 min 90% B, 12 min 90, return to initial conditions. |
|
MS system: |
ACQUITY Qda |
|
Ionization mode: |
ESI+ |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
10 V |
|
Desolvation temp.: |
600 °C |
|
Source temp.: |
150 °C |
|
MS scan range: |
100 to 1000 m/z |
|
Sampling rate: |
5 Hz |
The ACQUITY Arc System employs Arc Multi-flow path™ Technology which provides options for selectable dwell volume. This offers increased flexibility for chromatographic separations and maximizes productivity by accommodating 3.0-µm to 5-µm particles for HPLC methods, while also supporting rapid and efficient UHPLC separations using 2.5-µm to 2.7-µm particles.6 Chromatographic separation of the pesticide formulation sample was performed in UHPLC mode with a CORTECS C18+ Column (3.0 x 100 mm, 2.7-µm solid-core particle technology, part no. 186007402). CORTECS 2.7-µm Columns are compatible on HPLC and UHPLC instrumentation. These columns have high efficiency at HPLC backpressures, resulting in faster analyses with better resolution than current methods using 5 µm, fully porous particles.7
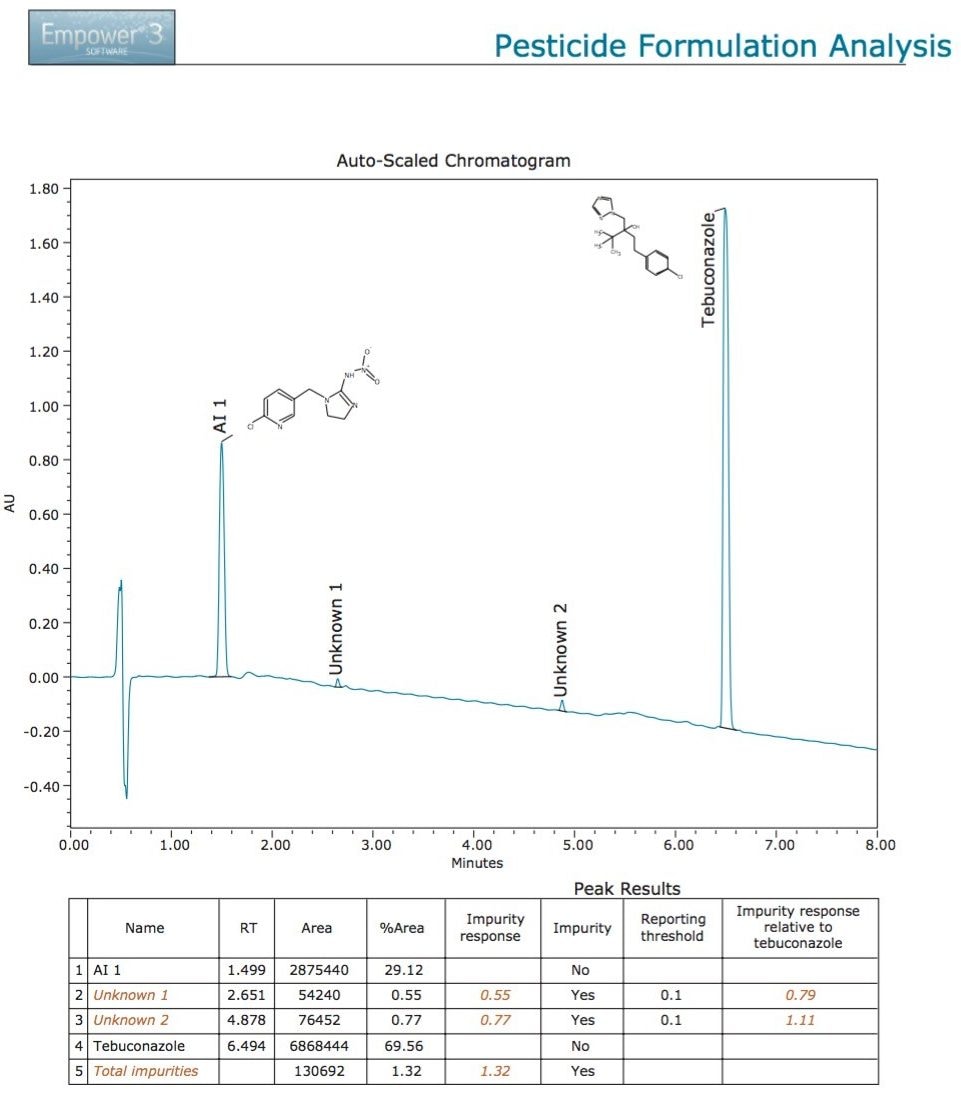
Following the analysis of the pesticide formulation and subsequent processing with Empower 3 Software, it was noted that there were two unknown components that exceeded the reporting threshold of 0.1%, which was set in the Empower processing method.8 The Empower report (Figure 2) shows the ACQUITY Arc UV chromatogram at 220 nm, resulting from the separation of the pesticide formulation sample. The two active ingredients have been identified and the unknown components have been labeled. Beneath the chromatogram, the peak results table displays the component name, area, area%, retention time, impurity response, and the reporting threshold. The UV and MS data suggested that Unknown 1 and Unknown 2 may share common structural features with the tebuconazole AI (Figure 3), therefore an Empower 3 Custom Calculation was employed to calculate the area% of each of the impurities relative to the tebuconazole (Figure 2 table). The tabulated data with the detected impurities highlighted in red makes it easy to interpret which components may require further investigation.
Combining chromatographic, UV, and mass detector results in a single place in Empower Software can further ease the burden of data interpretation. The Empower Mass Analysis window (Figure 3) provides a single location to associate chromatographic peaks from all of the detectors used in the analysis with their corresponding spectra. The UV chromatogram and spectra are displayed, along with the total ion chromatogram (TIC) and mass spectra with extracted ion chromatograms (XIC) also shown. The spectra from the detected peaks are time aligned and displayed in a window above the chromatograms facilitating rapid data review.
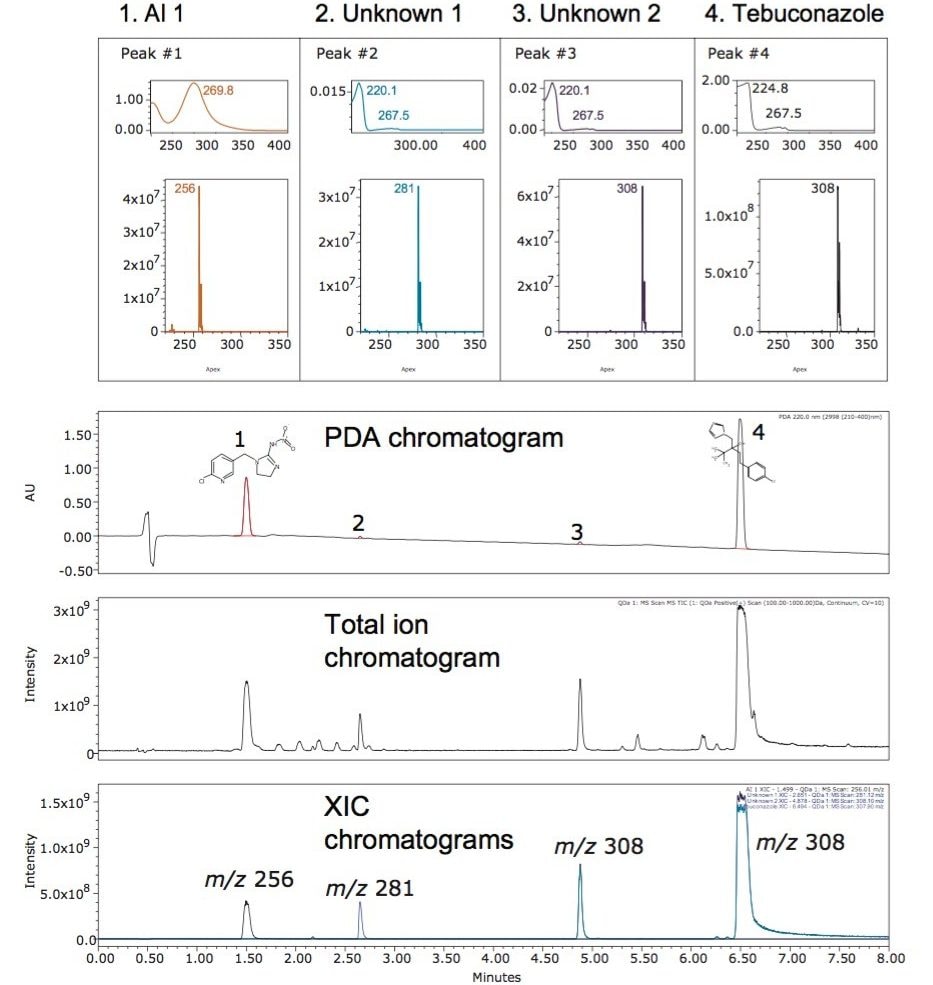
Interrogation of the data in the Mass Analysis window indicated relationships between Unknown 2 (Peak 3) and the tebuconazole AI (Peak 4). The UV spectra have similar absorption maxima while the mass spectra indicate that tebuconazole, with an [M+H]+ corresponding to m/z 308 and Unknown 2 share the same m/z. In addition, the isotopic pattern of Unknown 2 is typical of a chlorinated compound (see Figure 4) and is identical to that of the AI. In a single analytical injection, Unknown 2 has been identified as having a similar UV spectrum and the same m/z and isotopic pattern as tebuconazole. These results suggest that Unknown 2 is likely an isomer of tebuconazole, and possibly has related structural composition and chemical properties. In addition, the detection sensitivity is greatly improved for the unknown components in the mass chromatogram, especially when the ions corresponding to the peaks of interest have been extracted from the TIC. The increased detection sensitivity provided by the ACQUITY QDa enables improved confidence in the assessment of the data.
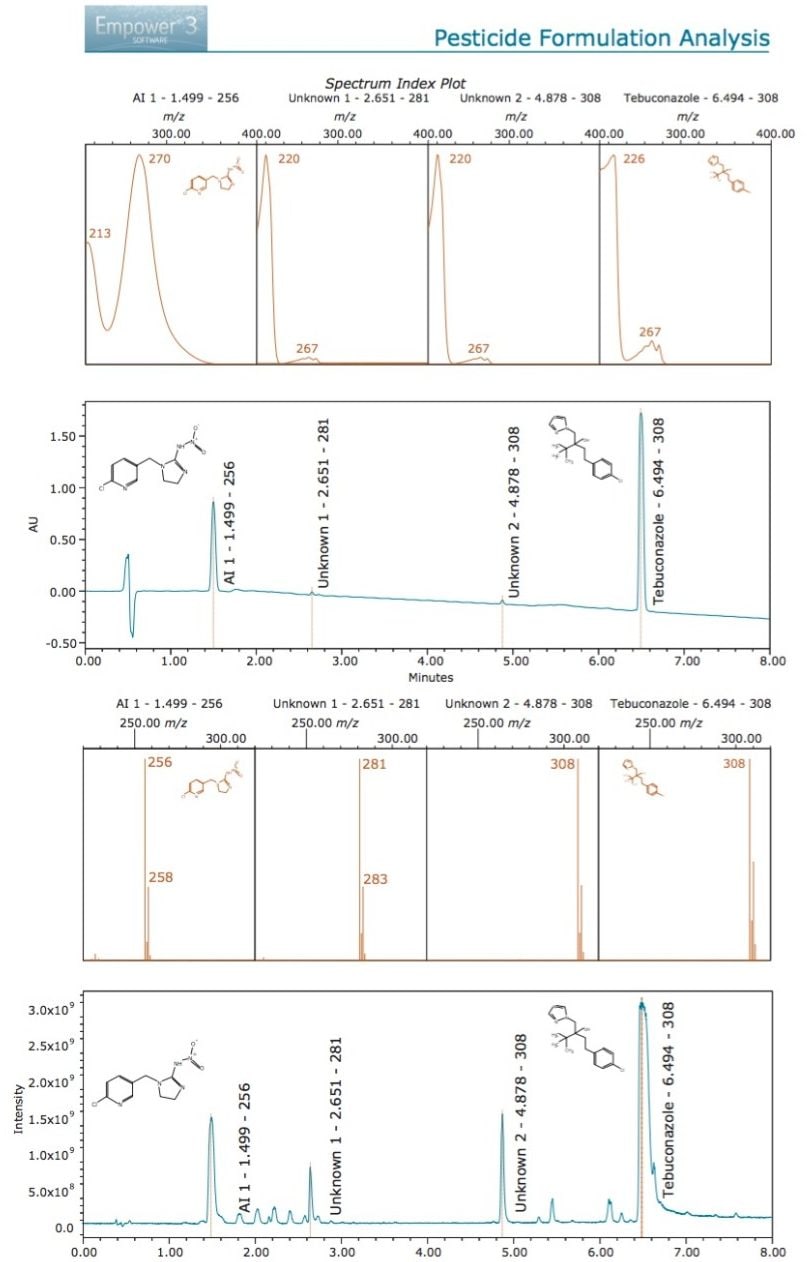
The UV maxima of Unknown 1 (Peak 2) showed close similarities when compared with that of Unknown 2 and tebuconazole which may indicate that the impurity is also related to this AI. The m/z for Unknown 1 is 281 and the isotopic pattern suggests chlorine in the structure (Figure 4). An Empower Spectrum Index Plot report summarizing the data is shown in Figure 4.
720005663, April 2016