This application brief describes how to assess relative performance in terms of resolution and peak capacity across instrument platforms (HPLC, UHPLC, and UPLC) using a preserved reversed-phase method for peptides.
Modern LC systems having lower system dispersion offer improved performance capabilities in the analysis of biomolecules.
Many methods used to analyze pharmaceuticals in late development and QC environments are HPLC-based, but there are notable advantages of modernizing analytical methods and instrumentation. Because biopharmaceuticals are inherently more complex than small molecules, analysis requires differentiating greater levels of molecular complexity and variation. Industry and regulators alike have come to recognize and support continual process improvement and adoption of new instrumentation in an effort to enhance overall product quality. By updating HPLC systems to lower dispersion LC platforms, legacy methods can be reproduced or updated to take advantage of greater performance capabilities as well as modern column technologies.
In this study, three quaternary LC platforms with known differences in system dispersion are evaluated using a reversed-phase gradient method. Although isocratic methods show a more noticeable difference in performance improvement with lower dispersion systems, gradient methods are more commonly used in the analysis of biotherapeutics. For this reason, peptide standards are used as a model analyte to demonstrate performance enhancements across the three systems when transitioning to lower dispersion systems. Empower 3 chromatography data software was used to integrate chromatograms and report peak width and resolution according to USP guidelines, which can be specified within the processing method.
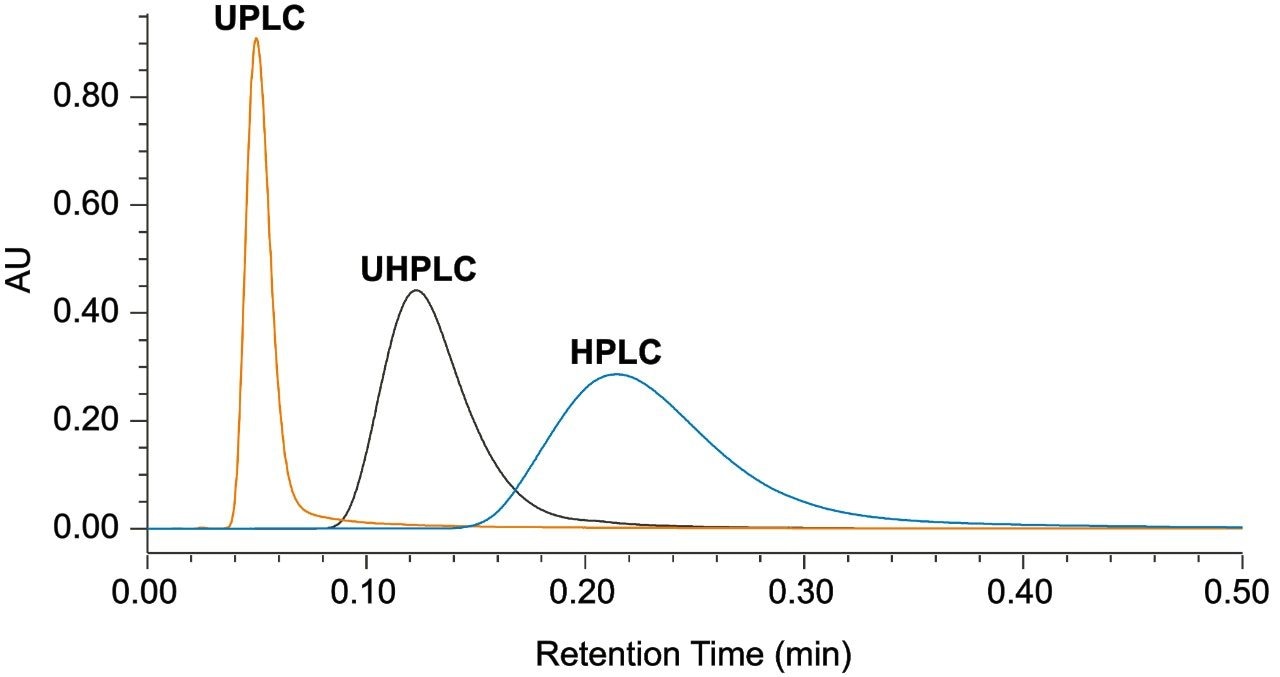
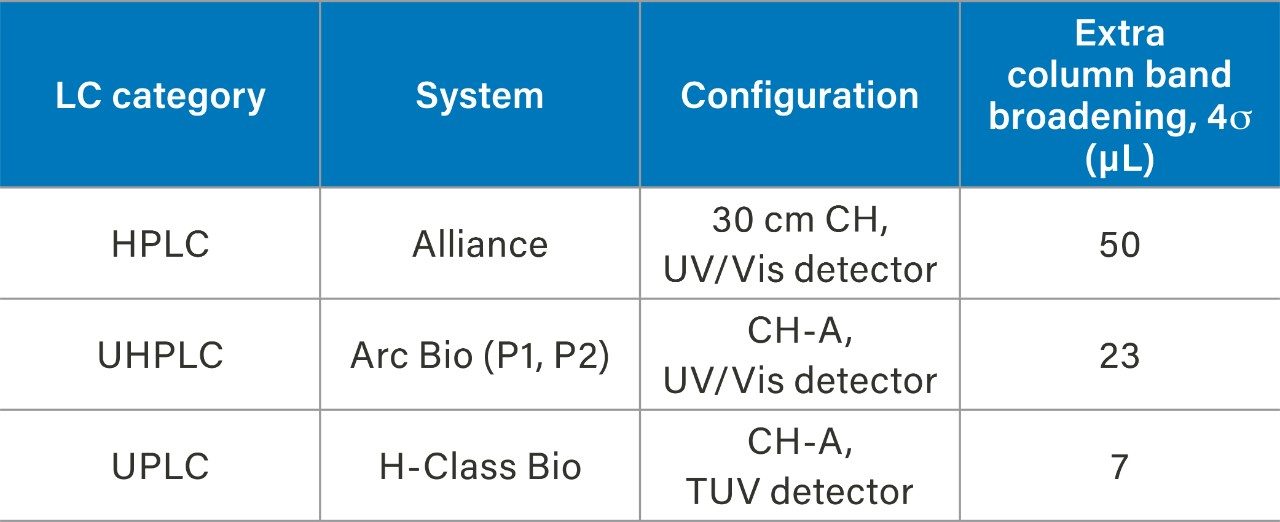
An HPLC system (Alliance), a bio-compatible UHPLC system (Arc Bio System), and a bio-compatible UPLC system (H-Class Bio System) were used to demonstrate performance improvements through instrument modernization, where the H-Class Bio System is the lowest dispersion system. To determine the extra column band broadening of each of the systems, a zero dead volume union was put in place of a column, and 30:70 water:acetonitrile was used as the mobile phase in an isocratic elution of a 1 µL injection of caffeine. An overlay of a representative injection from each of the three systems can be seen in Figure 1. By measuring the peak width (units of min) and multiplying by the flow rate, 0.3 mL/min, the extra column band broadening can be calculated and is reported in Table 1. Considerable improvement can be seen when transitioning from higher to lower dispersion instruments (Alliance>Arc Bio System>H-Class Bio System).
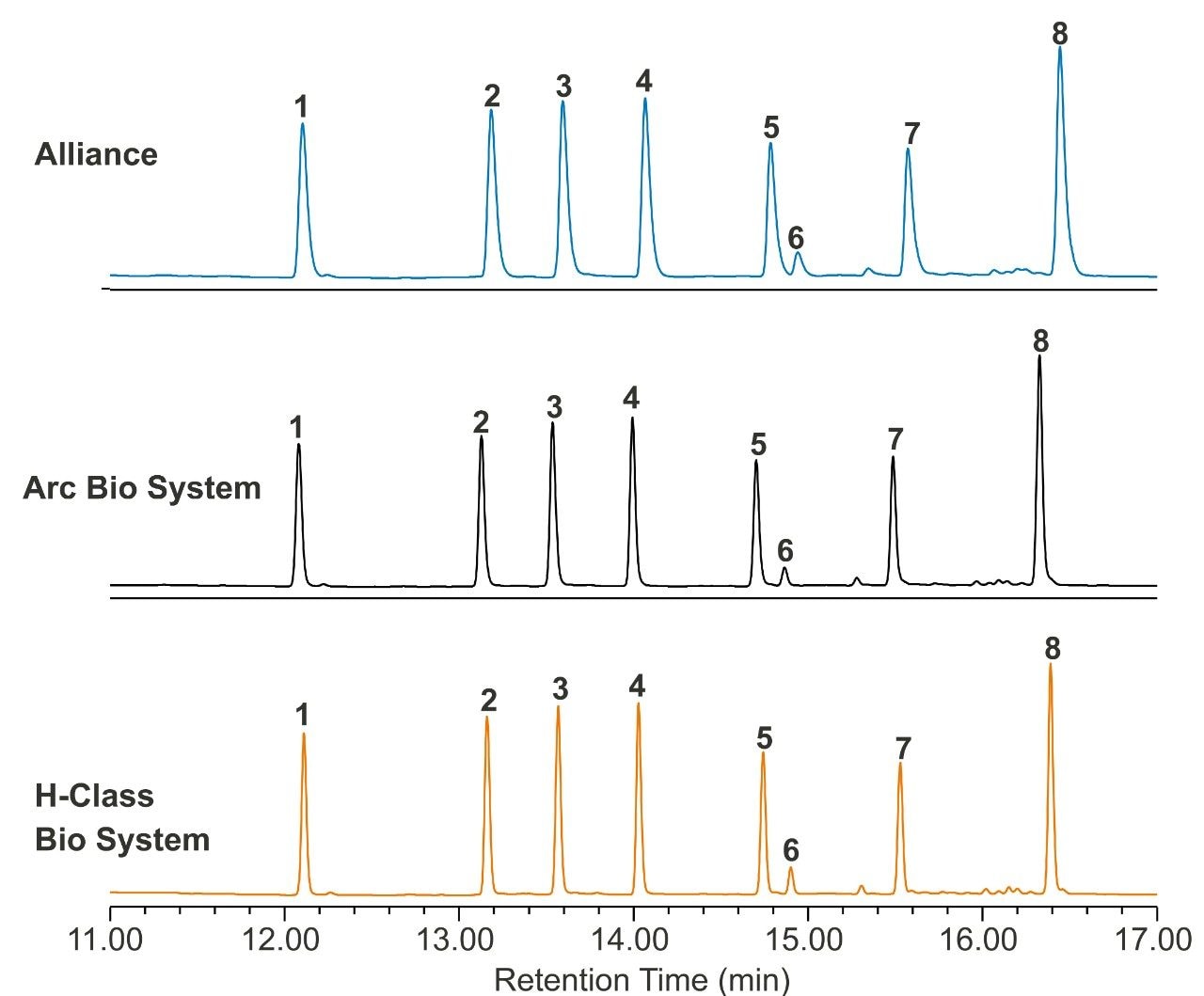
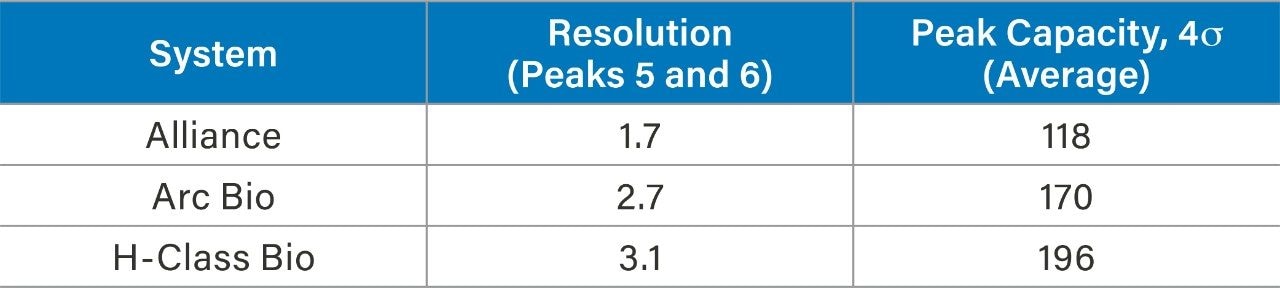
To explore how a lower dispersion system impacts performance of an analytical method, a reversed-phase gradient method for peptides was used to assess resolution and peak capacity across the systems. Peptide standards were selected as a model analyte to demonstrate proof of concept. A 10 minute gradient (5%–50% acetonitrile in TFA) using a Waters XBridge BEH C18 Column (130 Å, 2.5 µm, 4.6 x 100 mm) was run on each of the three systems, keeping method conditions unchanged. The resulting chromatography can be seen in Figure 2. The ACQUITY Arc Bio System and the H-Class Bio System generate narrower peaks, which results in higher peak capacity and better resolution. Peak capacity and resolution are reported in Table 2 for each of the three systems. Resolution between peaks five and six improved from 1.7 (HPLC) to 2.7 (UHPLC) and 3.1 (UPLC). Likewise, peak capacity increased from 118 (HPLC) to 170 (UHPLC) and 196 (UPLC). Because the same column and conditions were used on each system, any observed peak broadening can largely be attributed to differences in the physical components of each system that make up the flow path from the injector to the detector. In a more complex sample, such as a protein digest, lower dispersion systems could foreseeably resolve critical pairs with greater sensitivity. To further show the benefit of lower dispersion systems, legacy methods can also be updated to take advantage of narrower diameter columns with smaller particle sizes, whereas legacy instrumentation exceeds its pressure limitations.
Performance enhancements of lower dispersion LC systems were compared by evaluating resolution and peak capacity across HPLC, UHPLC, and UPLC instrumentation. Extra column band broadening was calculated for each system, and as expected, was significantly less for UHPLC (Arc Bio System) and UPLC (H-Class Bio System) platforms compared to an HPLC system (Alliance). The effects of lower dispersion systems were illustrated using a reversed-phase gradient method for peptides to demonstrate improved resolution and peak capacity across three LC platforms. From routine assays to method development of complex analytes, noted performance differences between instrument platforms offer a fit-for-purpose solution in the analysis of biomolecules.
720006275, May 2018