Pioneering strategies to incorporate ion mobility TWCCSN2 in pesticide screening assays have been presented,4-6 and the routine use of TWCCSN2 for small molecule analysis has evolved across multiple areas of research, including pharma (metabolism, metabolomics, and lipids), forensic toxicology, food safety (veterinary drugs, mycotoxins, steroids, steviol glycosides, natural product screening, and natural toxins).7-10 TWCCSN2 searchable libraries have been produced, where the TWCCSN2 value has been used as a screening parameter to improve the specificity of identification and decrease false detections. The approach has a chromatographic multi-additive method and MS library (incorporating TWCCSN2), which has been utilized to perform investigations into non-targeted screening of FAs in “off the shelf” food commodities.
Although the use of food additives (FAs) is strictly regulated under various European Union (EU) acts,1 national authorities have the responsibility to ensure effective controls are in place and monitor the consumption of food additives within their respective populations.2 To fulfil these requirements, analytical methods are required to be able to quantify these substances in a wide variety of foodstuff types for a large number of items available in the marketplace. Many analytical applications are already successfully implemented but generally cover very few additives and/or few food matrices. Under these conditions, it is very challenging and expensive to monitor additive levels in foods, considering the large availability of products on the market. An approach to make the analytical process more efficient can be achieved through the development of more versatile, high-throughput multimethods, which can flexibly cover the largest number of FAs in one analysis. Such methods will promote better coverage of additive composition for foods that are required to be controlled, and additionally can be used for exposure assessment to multiple FAs with a single analysis per sample.
According to the EU legislation, FAs are “any substance not normally consumed as a food in itself and not normally used as a characteristic ingredient of food, whether or not it has nutritive value.”3 These substances are authorized for use in food by the European Commission after being subjected to a safety assessment by the European Food Safety Authority (EFSA). Authorization is dependent upon no observation of health hazards and whether use complies with EU legislation (e.g., technological need and the benefits for consumers). Enforcement of the legislation through implementation of national food control systems should ideally cover all food marketed within the country. Likewise, for risk assessment, the analysis of large numbers of products are necessary in order to obtain a representative estimate of the daily intake of FAs. To address the increasing number of sample matrices and the large number of FAs (authorized/unauthorized), it is essential to develop effective and reliable analytical methods.
We have investigated the utility of mass spectrometry libraries incorporating a TWCCSN2 (travelling wave collision cross section against nitrogen buffer gas) metric. UPLC-IM-MS (UltraPerformance Liquid Chromatography ion mobility mass spectrometry) is comprised of ion mobility spectrometry (IMS; gas phase separation prior to MS analysis) coupled with UPLC (neutral species separation). The nested timescales of UPLC (seconds), IMS (~10 milliseconds), and time-of-flight MS (microseconds) are compatible with the requirement of high-throughput analysis of complex samples. IM separation of compounds results from gas phase ions being separated within a travelling wave ion mobility (TWIM) RF ion guide, located prior to the mass analyser of the instrument. Mobility separation is obtained by driving packets of ions through a low-pressure inert buffer gas (typically nitrogen) using a relatively weak electric field. The resultant separation depends on factors such as the mass, charge, and shape of the molecule. It provides an added dimension of separation to that of LC and MS, in addition to generating TWCCSN2 as a complementary identification metric.
Pioneering strategies to incorporate ion mobility TWCCSN2 in pesticide screening assays have been presented,4-6 and the routine use of TWCCSN2 for small molecule analysis has evolved across multiple areas of research, including pharma (metabolism, metabolomics, and lipids), forensic toxicology, food safety (veterinary drugs, mycotoxins, steroids, steviol glycosides, natural product screening, and natural toxins).7-10 TWCCSN2 searchable libraries have been produced, where the TWCCSN2 value has been used as a screening parameter to improve the specificity of identification and decrease false detections. The approach has a chromatographic multi-additive method and MS library (incorporating TWCCSN2), which has been utilized to perform investigations into non-targeted screening of FAs in “off the shelf” food commodities.
Food commodities screened for food additives: Red fruits yogurt (YB); strawberry yogurt (YS); energy drink (D1); “zero” lemon drink (D2); “zero” strawberry and kiwi drink (D3); colorless tonic drink (D4); and sparkling lemonade drink (D5). (Note: zero equals no added sugars.)
Yogurt extraction method: Yogurt samples (15 g) were weighed into Waters 50-mL screw-cap centrifuge tubes. A 10-mL volume of acetonitrile in 1% acetic acid was added as an extraction solvent and the tube then mixed vigorously for one minute using a vortex mixer. Anhydrous MgSO4 (6 g) and sodium acetate (1.52 g) were added to the tube to induce phase separation. Samples were immediately shaken for one minute, and then centrifuged for five minutes at 1500 rcf at 4 °C. Dispersive-SPE (dSPE) of the samples was carried out by pouring the supernatant (8 mL) into a centrifuge tube (50 mL) containing MgSO4 (1.2 g), PSA (410 mg), and C18 (404 mg). The sample was vortexed for one minute and centrifuged for five minutes at 1500 rcf at 4 °C.
|
LC system: |
ACQUITY UPLC I-Class PLUS |
|
Detection: |
Ion mobility mass spectrometry |
|
Vials: |
LCMS Certified Clear Glass 12 × 32 mm Screw Neck Total Recovery Vial, with Cap and Pre-slit PTFE/Silicone Septa, 1 mL volume (p/n: 600000671CV) |
|
Column: |
ACQUITY UPLC HSS T3 100 mm × 2.1 mm, 1.8 μm (p/n: 186003539) |
|
Column temp.: |
45 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
Water with 10 mM ammonium acetate (0.1% formic acid) |
|
Mobile phase B: |
Methanol/acetonitrile (1:1) with 10 mM ammonium acetate (0.1% formic acid) |
|
Gradient: |
0–0.5 min isocratic at (95:5(A:B)); 6.0 min (0:100); 9.0 min (0:100); 9.5 min (95:5) 11.0 min (95:5) |
|
MS system: |
SYNAPT G2-Si |
|
|
Ionization mode: |
ESI+ and ESI |
|
|
Capillary voltage: |
3 kV(ESI+) and 2.2 kV(ESI-) |
|
|
Cone voltage: |
30 V |
|
|
Desolvation temp.: |
550 °C |
|
|
Source temp.: |
150 °C |
|
|
Acquisition range: |
m/z 50–1200 |
|
|
Acquisition rate: |
10 spectra per second |
|
|
Lock mass: |
Leucine enkephalin (C28H37N5O7 (m/z 556.2766 +ve) and (m/z 554.2620 -ve)) |
|
|
Collision energy: |
HDMSE low collision energy 4 eV and high collision energy ramp (10–45 eV) |
|
|
MS resolution: |
20,000 resolution full width half maximum (FWHM) at m/z 556 |
|
|
IM resolution: |
≈40 Ω/ΔΩ (FWHM) |
|
|
IMS parameters: |
Default IMS screening parameters include T-Wave Velocity Ramp = Start: 1000 m/s and End: 300 m/s; T-Wave Pulse Height = 40 V; and a gas flow of helium 180 mL and nitrogen 90 mL (buffer gas) for the respective gas cells was used, giving an IM cell pressure of ~3.2 mBar |
|
|
Calibration: |
IMS/ToF Calibration Kit (p/n:186008113) |
|
|
Chromatography software: |
MassLynx v4.1 SCN 916/924 |
|
MS software: |
MassLynx v4.1 SCN 916/924 |
|
Informatics: |
MassLynx data post-processed using UNIFI v1.94 |
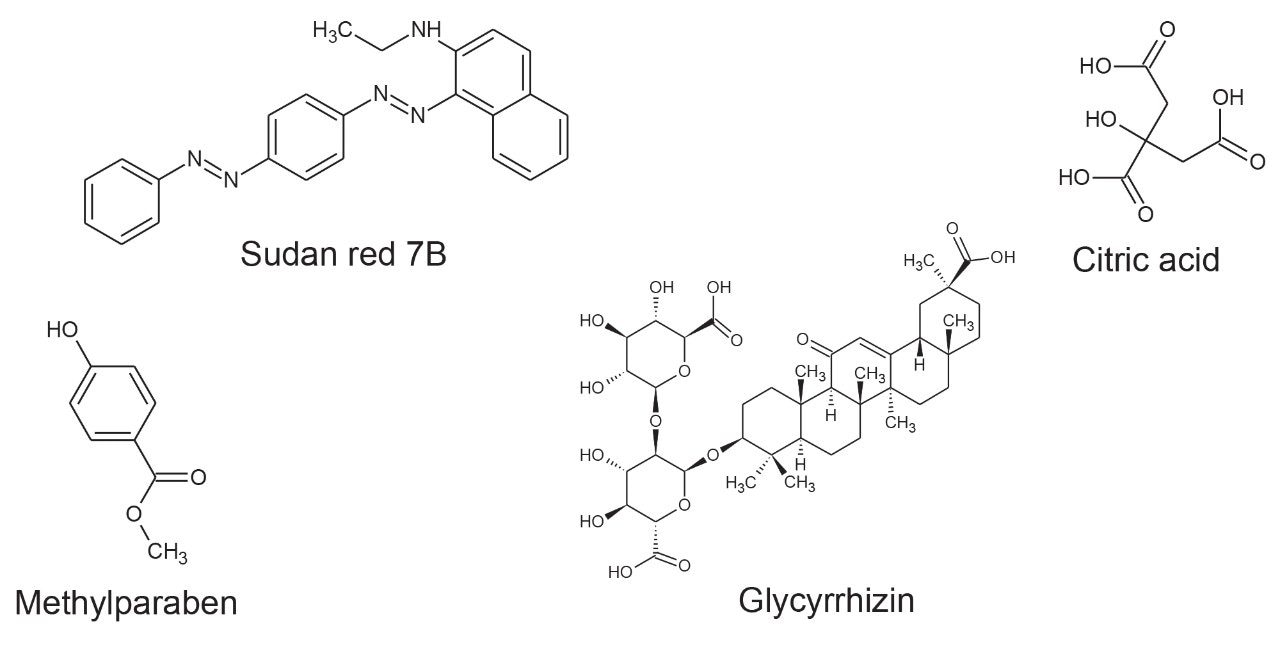
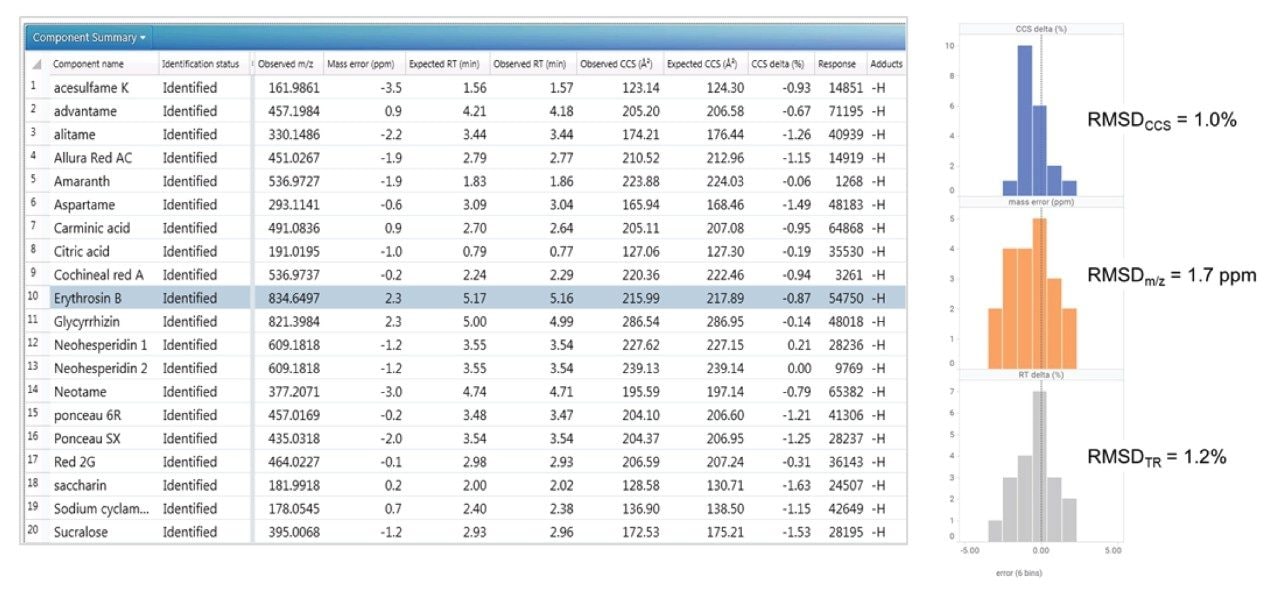
Positive and negative ion mass spectrometry libraries for LC-MS-amenable food additives were developed using a standardized library generation protocol.11 The strategy determines both precursor ion, ion mobility product ions, and collision cross section values. The generated library contains data for food additive classes such as colorings, preservatives, antioxidants, and sweeteners, including the banned sweetener glycyrrhizin. Examples of the classes of food additive characterized using ion mobility are shown in Figure 1.
Seven “off-the-shelf” food samples labelled as containing a variety of FAs, including sweeteners, preservatives, and food colorings, were purchased from Belgian supermarkets. The sample analysis performed using the FA multi-method was used to test the robustness of the TWCCSN2 library generated. UPLC HDMSE data were acquired in positive and negative ion modes, enabling comparison of the precursor/ion mobility product ions and TWCCSN2 incorporated in the food additives libraries.
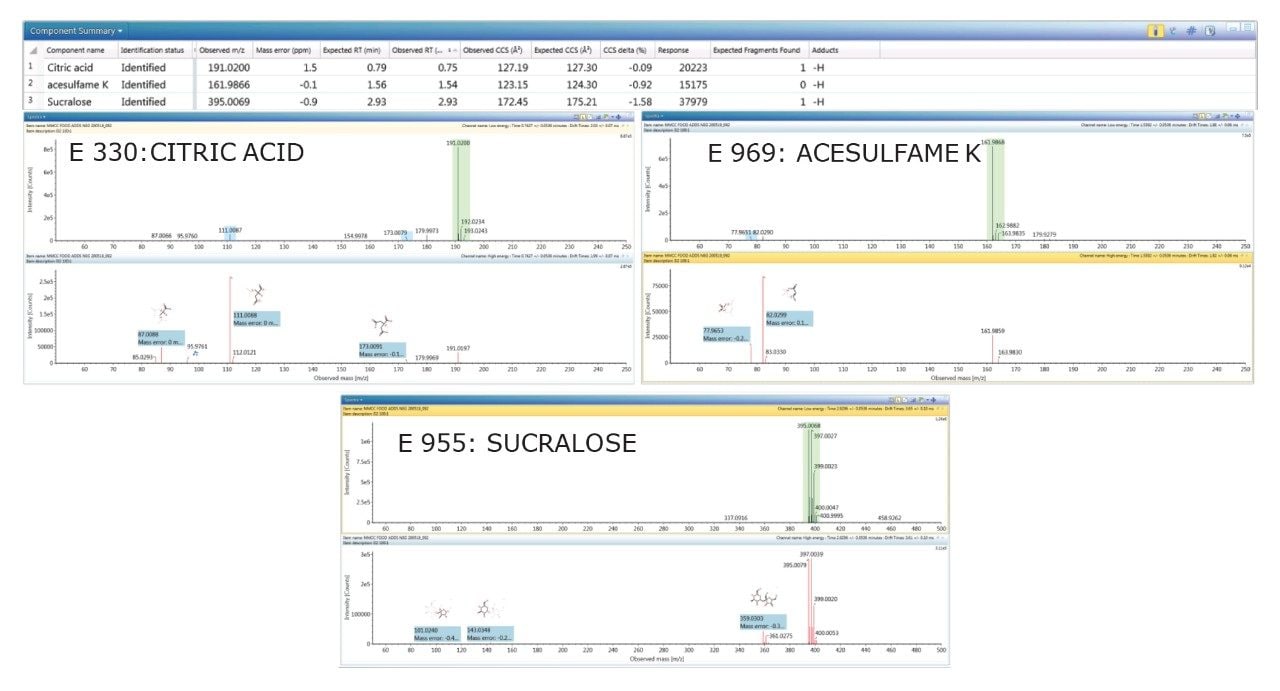
Using a “blind test” strategy for food commodity D2 (colorless lemon soft drink), two sweeteners (acesulfame E 969 and sucralose E 955), and a food preservative (citric acid E330) were detected and positively identified, with accurate mass measurement of 2 ppm and TWCCSN2 Δ <2%. No food additive colorings were observed in sample D2. The corresponding negative ion HDMSE precursor ion/mobility product ion spectra and CCS values for the food additives and natural constituent hesperidin identified in sample D2 are presented in Figure 2.
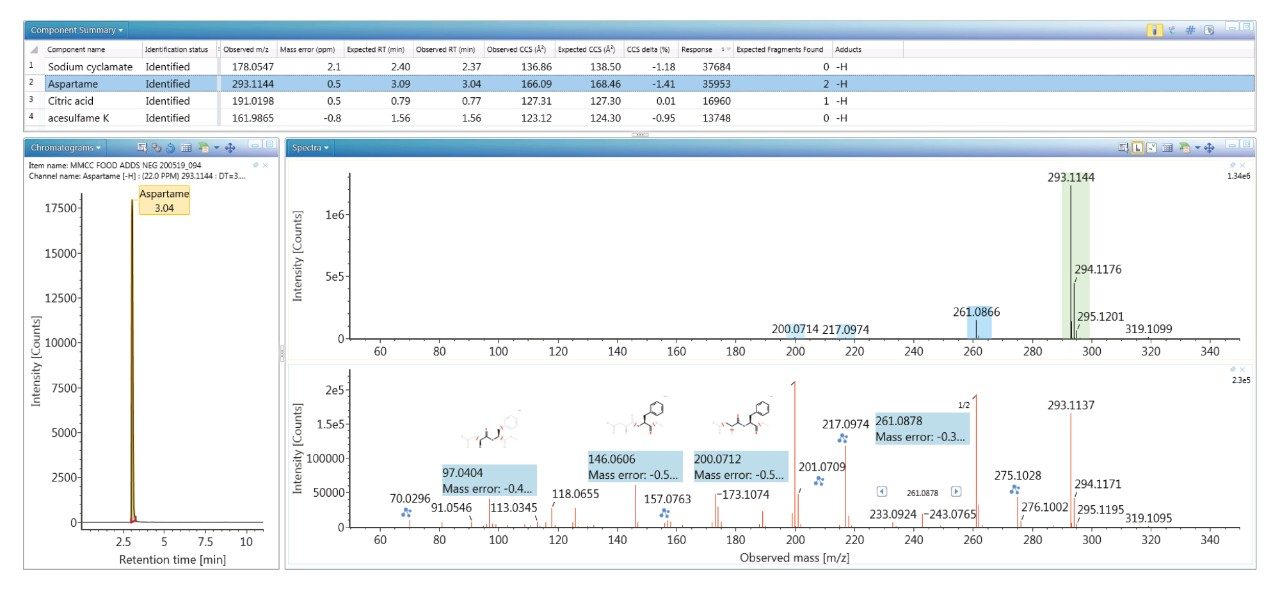
The value of the TWCCSN2 libraries generated is illustrated in Figure 3 for food coloring (sodium cyclamate E 952), antioxidant (citric acid E 330), and sweetener (aspartame E 951 and acesulfame K E 950) agents identified in food commodity D3 (strawberry and kiwi drink), with the corresponding negative ion HDMSE ion mobility precursor/ion mobility spectra for aspartame. The highly specific non-targeted retention time/drift time aligned ion mobility product ion spectrum obtained for aspartame shows product ion mass accuracy within 1 mDa (m/z 97.0404 =-4.12 ppm, m/z 146.0606=-3.4 ppm, m/z 200.0712=-2.5 ppm, m/z 261.0878=-1.14 ppm). For the food additives identified, delta TWCCSN2 Δ <2% were observed. Moreover, no false detections were obtained for the food commodities screened using the food additive TWCCSN2 MS library.
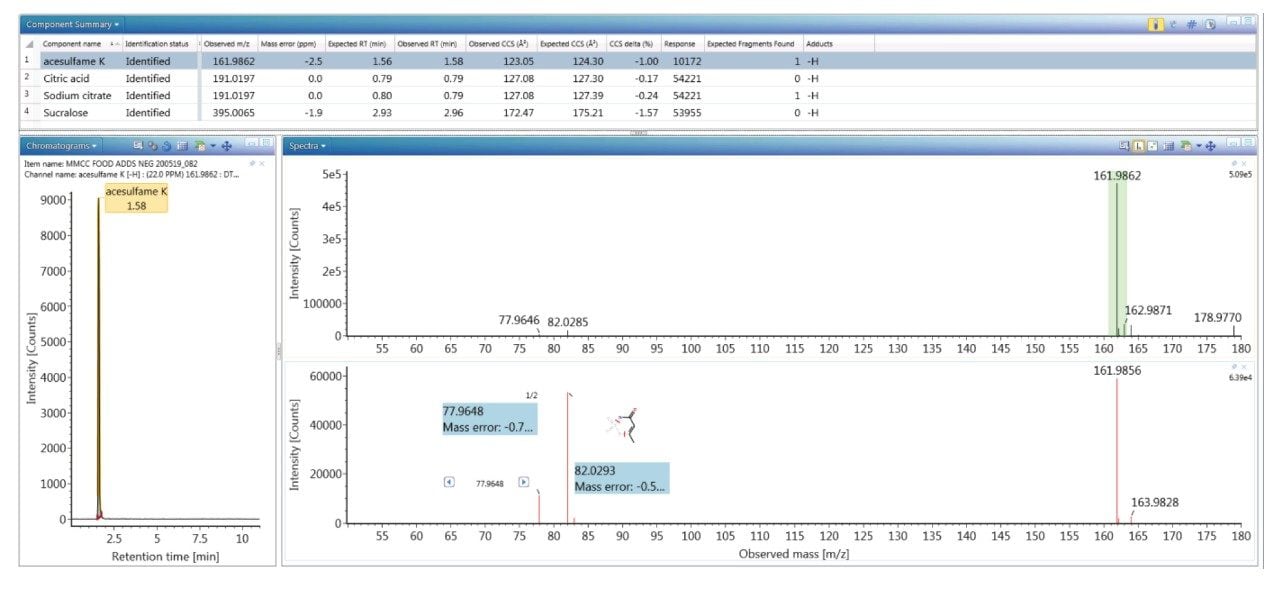
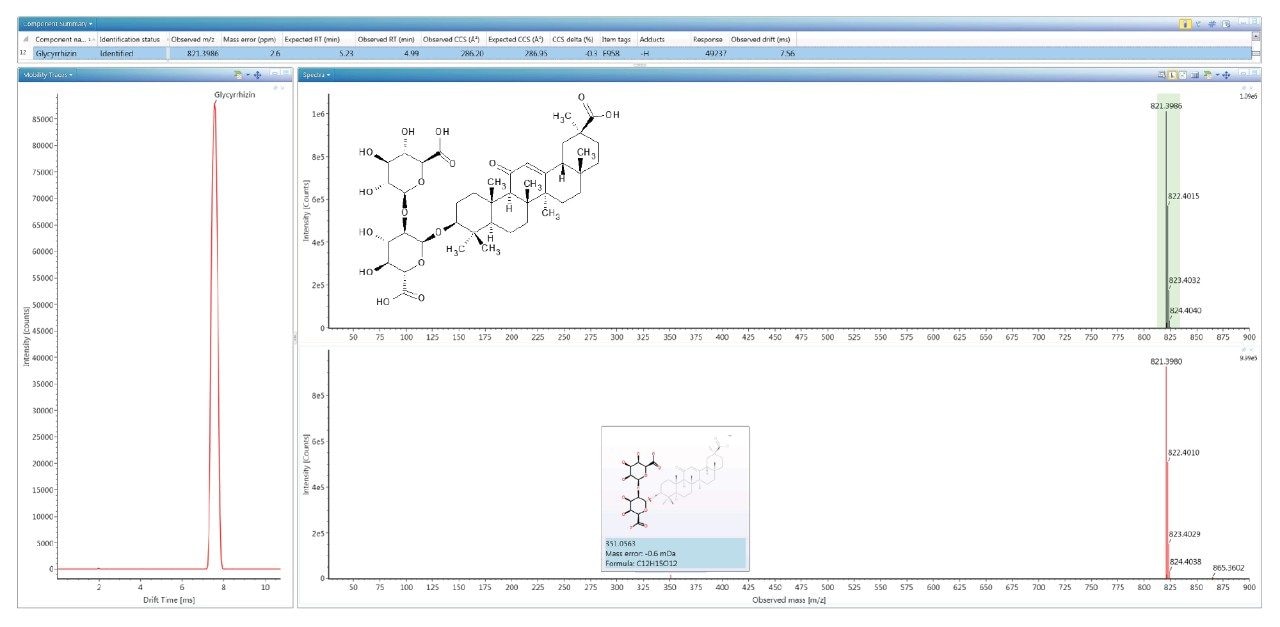
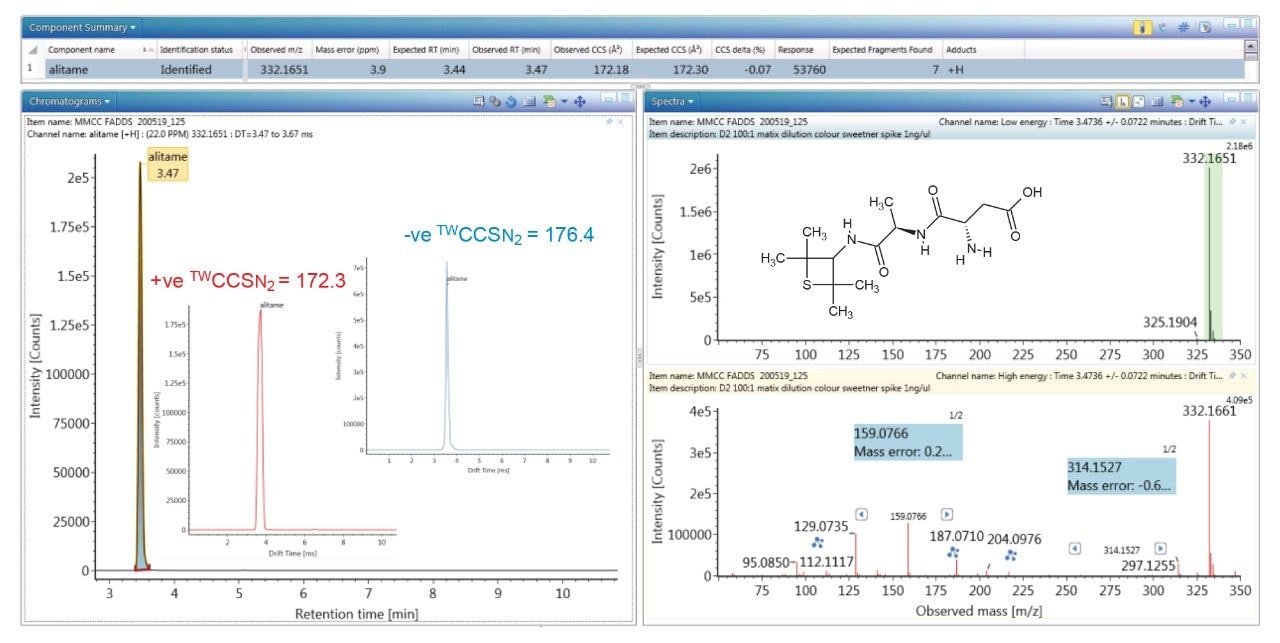
To evaluate the robustness of the library when used in screening, several food commodities were also spiked with a series of food additive colorings and sweeteners. Food commodity D4 is a colorless tonic drink for which it would be expected that that no food coloring additives would be detected, as can be seen from the results shown in Figure 4, where the negative ion HDMSE precursor/ product ion spectra for additive acesulfame K (E 950) is shown, as well as the detection of compounds E 330 and E 955. The tonic drink D4 was spiked with a series of additional sweeteners and colorings for which the component summary detection results are shown in Figure 5, illustrating that the unauthorized sweeteners, alitame and glycyrrhizin were correctly detected. The ion mobility trace and HDMSE precursor/product ion spectra for detection glycyrrhizin (E 958) are shown in Figure 6, where for glycyrrhizin TWCCSN2=286.2 Å2 (TWCCSN2 Δ = -0.3%), was obtained. In Figure 7, the positive ion HDMSE precursor and ion mobility product ion spectra for food additive library constituent alitame (E 956) is presented, with the +ve and -ve mobility traces and respective 172.3 Å2/176.4 Å2 values measured. The combined specificity of these values of these characterized TWCCSN2 values can be used to confirm detection at trace levels where only monisotopic information is determined. The obtained results confirm the robustness of the FAs libraries, where expected and unexpected food additives can be screened for by using the combination of retention time, precursor/ion mobility product ion m/z, and CCS values. When compared to the MS library, CCS measurements are routinely determined with delta values <2% (compared to typically accepted MRM ratio screening tolerances of 20%), providing confidence that TWCCSN2 can be utilised as a robust and reliable identification metric, in conjunction with retention time and m/z, in the application of flexible screening data processing workflows within critically important food safety research studies.
720006768, February 2020