Determination of Pesticide Residues in Cucumber Using GC-MS/MS With APGC™ After Extraction and Clean-up Using QuEChERS
Abstract
Reliable analytical methods are needed for detection, quantification, and identification of hundreds of pesticide residues in many different commodities. This application note describes the development and validation of a comprehensive method based on gas chromatography-tandem mass spectrometry (GC-MS/MS) for the determination of over 200 pesticides. Extracts of cucumber were prepared using a version of the Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) method, including a dispersive solid-phase extraction (dSPE) step, followed by determination with GC-MS/MS. The use of GC-MS/MS utilizing atmospheric pressure gas chromatography (APGC) has been shown to offer significant improvements in performance over electron ionization (EI) for pesticide residue analysis, in terms of selectivity and specificity. The extremely high sensitivity of the APGC Xevo™ TQ-XS System was demonstrated with reliable detection for all the analytes at concentrations as low as 0.001 mg/kg, even when the injection volume was 1 µL. The method was successfully validated in cucumber using the SANTE guidelines document. The results from analysis of the spikes showed that almost all the analytes were within the required tolerance for recovery and repeatability, respectively. The method is considered sensitive, specific, accurate, and suitable for the determination of residues of a wide range of GC-amenable pesticides in agricultural commodities, for checking compliance with maximum residue levels (MRLs) and has the potential for determination at much lower concentrations.
Benefits
- The APGC System generates extremely high sensitivity to help meet the needs of those involved with the analysis of foods for pesticide residues
- Sufficient sensitivity was achieved using splitless injection of 1 µL of acetonitrile extract
- APGC adds flexibility to the laboratory as the same tandem mass spectrometry (MS/MS) system can also operate in combination with UPLC™
Introduction
Plant protection products, more commonly known as pesticides, are used to control pests, weeds, and diseases. Pesticide residues resulting from the use of such products on crops that are used for food or feed production may pose a risk factor for public health or hinder trade. MRLs or tolerances are established in raw agricultural commodities. Some countries (e.g. EU and Japan) operate a system of “default MRL”, equal to the limit of quantification (LOQ) achievable with analytical methods used for MRL enforcement, which is applicable for pesticides not explicitly mentioned in the MRL legislation. The value of this default MRL is typically 0.01 mg/kg. Compliance with MRLs is checked by the monitoring for residues in produce. Governments typically operate pesticide residue testing programs and the food industry and contract testing laboratories also carry out testing to check the levels of pesticide residues in agricultural commodities, ingredients, and finished food products.
Reliable analytical methods are needed for detection, quantification, and identification of hundreds of pesticide residues in many different commodities. One main driver for all laboratories involved with the determination of pesticide residues in food is to accurately determine the compounds of interest, at relevant concentrations, in the most cost-effective manner. Laboratories are constantly having to address issues with capacity and efficiency to address sample throughput requirements and to attain lower and lower reporting limits. The implementation of multiresidue methods, relying on generic extraction with limited clean-up (e.g. QuEChERS) and determination using both gas and liquid chromatography coupled with MS/MS has made a significant contribution to not only extending the scope of analyses but their effective and efficient implementation.
Laboratories carrying out pesticide residue analysis are always going to need gas chromatography (GC) to complement liquid chromatography (LC) to cover the scope of analytes required. GC is a powerful technique for the determination of the more volatile, nonpolar, and low polarity pesticides. The determination of GC-amenable pesticides in food using MS/MS allows for high selectivity and sensitivity and minimizes chromatographic interferences. The most common ionization technique for GC-MS/MS is EI, as it enables the determination of a wide range of organic compounds. However, extensive fragmentation results in reduced sensitivity as the ion current is distributed over many ions with poor intensity, as well as a low selectivity due to the formation of less specific fragment ions. The use of GC-MS/MS utilizing APGC has been shown to offer significant improvements in performance over EI for pesticide residue analysis, in terms of selectivity and specificity.1–4 Selectivity and sensitivity are enhanced if either the molecular/protonated ion or a high mass fragment ion are selected as the precursor ion for MRM transitions in MS/MS.
The objective of this study was to demonstrate the performance of a method for the determination of pesticide residues using GC-MS/MS with APGC on Xevo TQ-XS after QuEChERS. QuEChERS is a versatile, streamlined approach using rapid solvent-based extraction in a centrifuge tube, often followed by dSPE for clean-up, which is suitable for coupling with analysis of extracts by GC-MS/MS.
Experimental
Sample Preparation, Extraction, and Clean-up
Samples of cucumber were purchased from a local retail store. They were immediately homogenized in a food processer and stored frozen until required. In addition, a Quality Control Material of cucumber puree (T19290QC) was purchased from FAPAS. Samples were extracted using the CEN QuEChERS method.5 An overview of the details of the sample extraction and clean-up procedure used is given in Figure 1.
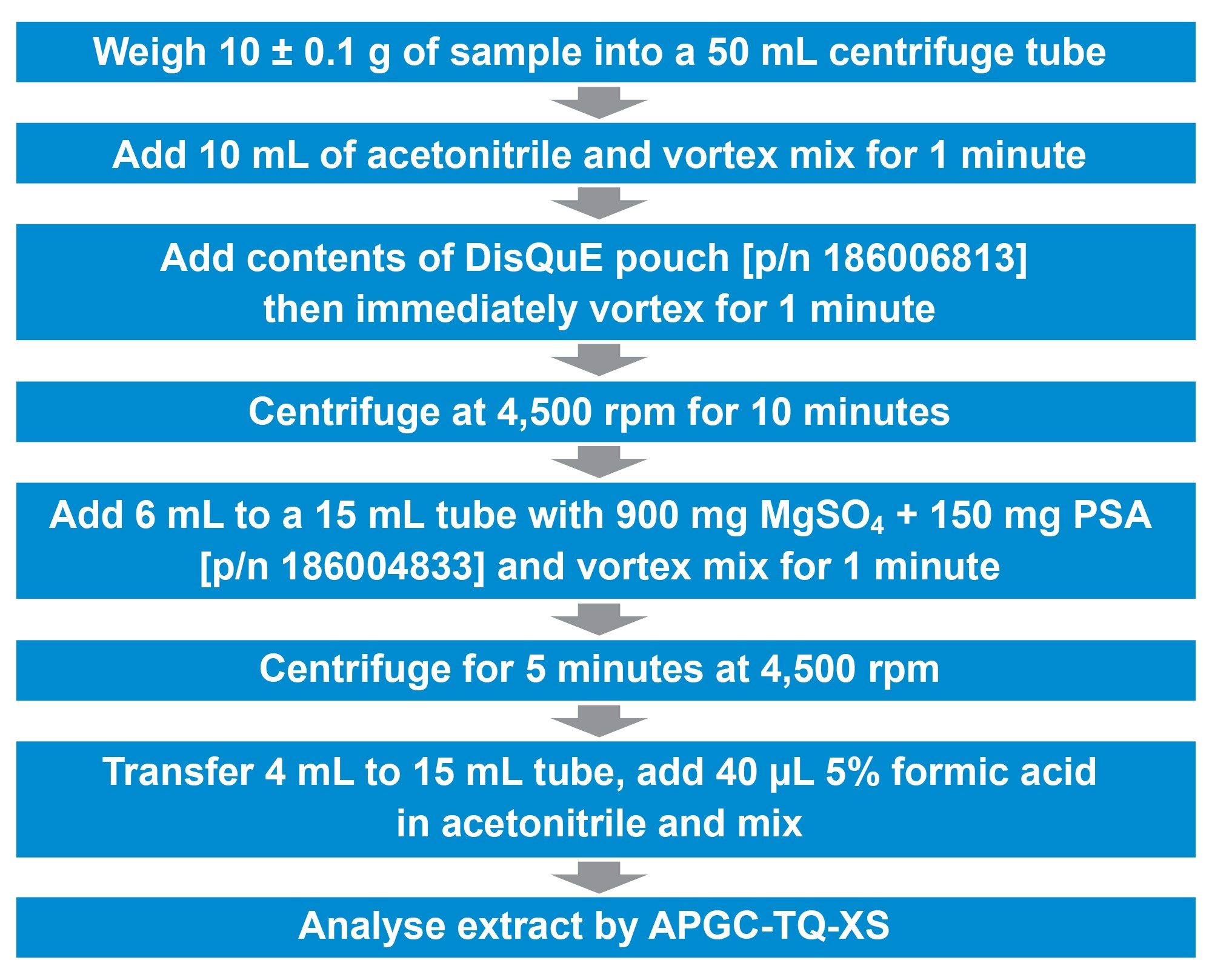
The GC Multiresidue Pesticide Kit (Restek pn 32562) was used to prepare working solution to create matrix-matched calibration standards and for spiking the cucumber test portions. The calibration standards were prepared over the range 0.0005 to 0.10 mg/kg.
GC Conditions
|
GC system: |
Agilent 7890A |
|
Autosampler: |
CTC CombiPal |
|
Wash solvent(s): |
Wash 1: ethyl acetate Wash 2: acetonitrile |
|
GC column: |
Restek Rxi-5Sil MS (30 m x 0.25 mm id x 0.25 µm film) |
|
Carrier gas: |
Helium |
|
Oven program: |
90 °C for 1 min, ramp to 330 °C at 8.5 °C/min hold for 5 min |
|
Gas flow rate: |
2 mL/min (constant flow mode) |
|
Injection type: |
Pulsed splitless |
|
Inlet temperature: |
250 °C |
|
Pulse time: |
1.2 min |
|
Pulse pressure: |
32 psi |
|
Purge flow: |
30 mL/min |
|
Septum purge flow: |
3 mL/min |
|
Inlet liner: |
Restek Topaz 4.0 mm ID Single Taper Inlet Liner w/ Wool |
|
Injection volume: |
1 µL |
|
Makeup gas: |
Nitrogen at 350 mL/min |
|
Transfer line temperature: |
280 °C |
MS Conditions
|
Mass spectrometer: |
Xevo TQ-XS |
|
Source type: |
APGC 2.0 with water as a modifier |
|
Source temperature: |
150 °C |
|
Transfer line temperature: |
280 °C |
|
Corona current: |
2.0 µA |
|
Auxiliary gas flow: |
200 L/hr |
|
Cone gas flow: |
265 L/hr |
Data Management
|
MS acquisition software: |
MassLynx™ v4.2 |
|
Quantitation software: |
TargetLynx™ XS |
The GC-MS/MS method for 203 pesticides and their metabolites was created using the Quanpedia™ Database, which automatically creates the MS acquisition method and processing method from a compendium of compound specific MS parameters such as transitions, and collision energy. A list of the compounds included in the method can be found in the Annex.
Method Validation
Validation was performed by replicate analysis of spiked cucumber. The following factors were assessed: selectivity, sensitivity, calibration graph characteristics, recovery, and within-laboratory repeatability (RSDr). Recovery and repeatability were determined from the analysis of five replicates prepared at two concentrations: the EU default MRL (0.01 mg/kg) and at 10 times lower concentration (0.001 mg/kg). In addition, five replicates of the Quality Control Material (T19290QC) were prepared, analyzed and results compared to the assigned value provided by FAPAS.
Results and Discussion
Figure 2 shows the chromatography for a selection of analytes. The dwell times were automatically calculated whilst ensuring at least twelve data points for each peak for precise measurements.
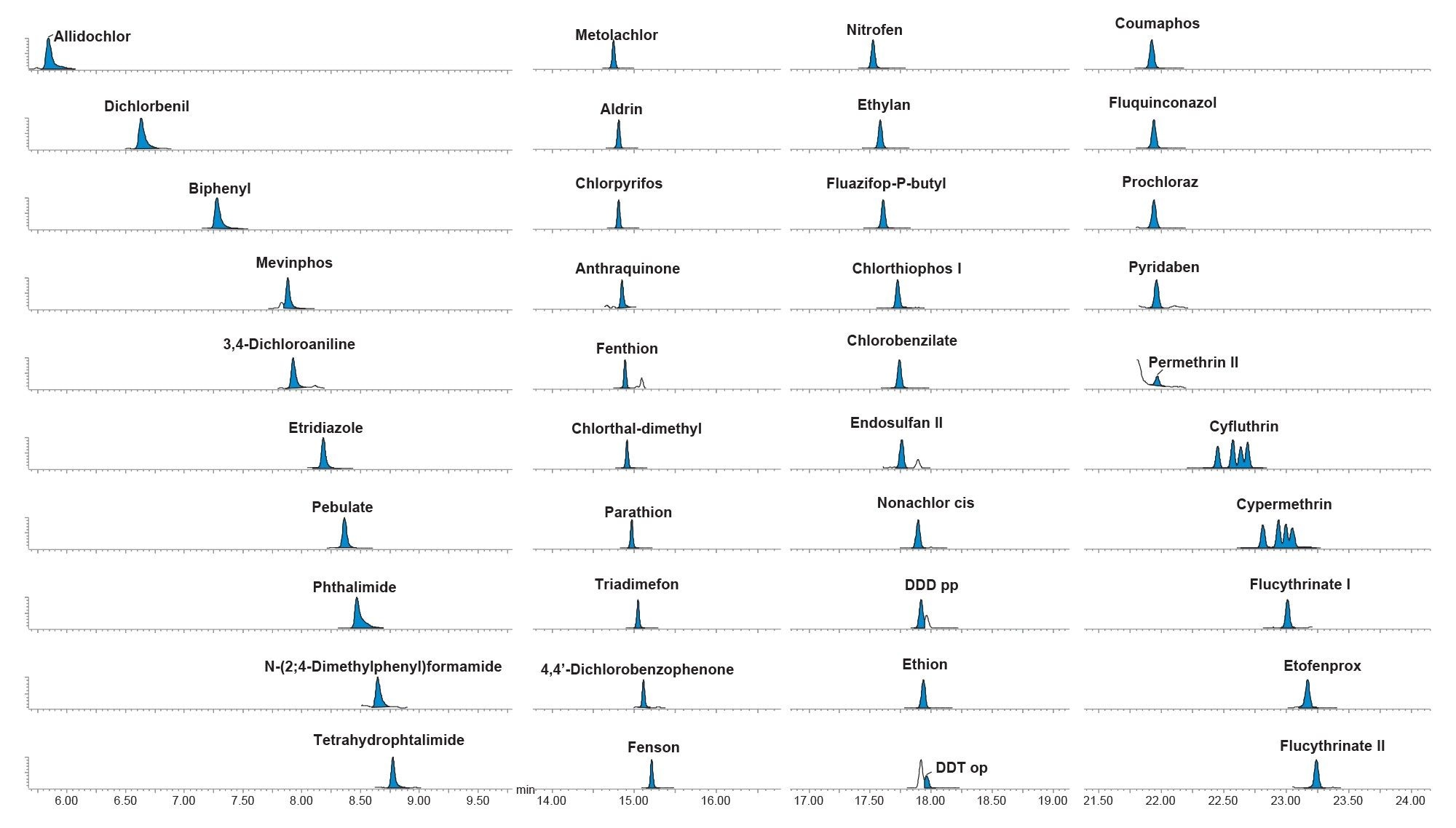
Acetonitrile has a large expansion volume, which limits the injection volume that can be used with conventional splitless injection and impacts sensitivity. Although this issue can be avoided by either using solvent exchange into another solvent such as toluene or by switching to a different design of injector, the programmable temperature vaporizer (PTV) with solvent vent, the capability to achieve sufficient sensitivity using a 1 µL injection of acetonitrile, with an easy-to-use conventional splitless injection unit, is an attractive option.
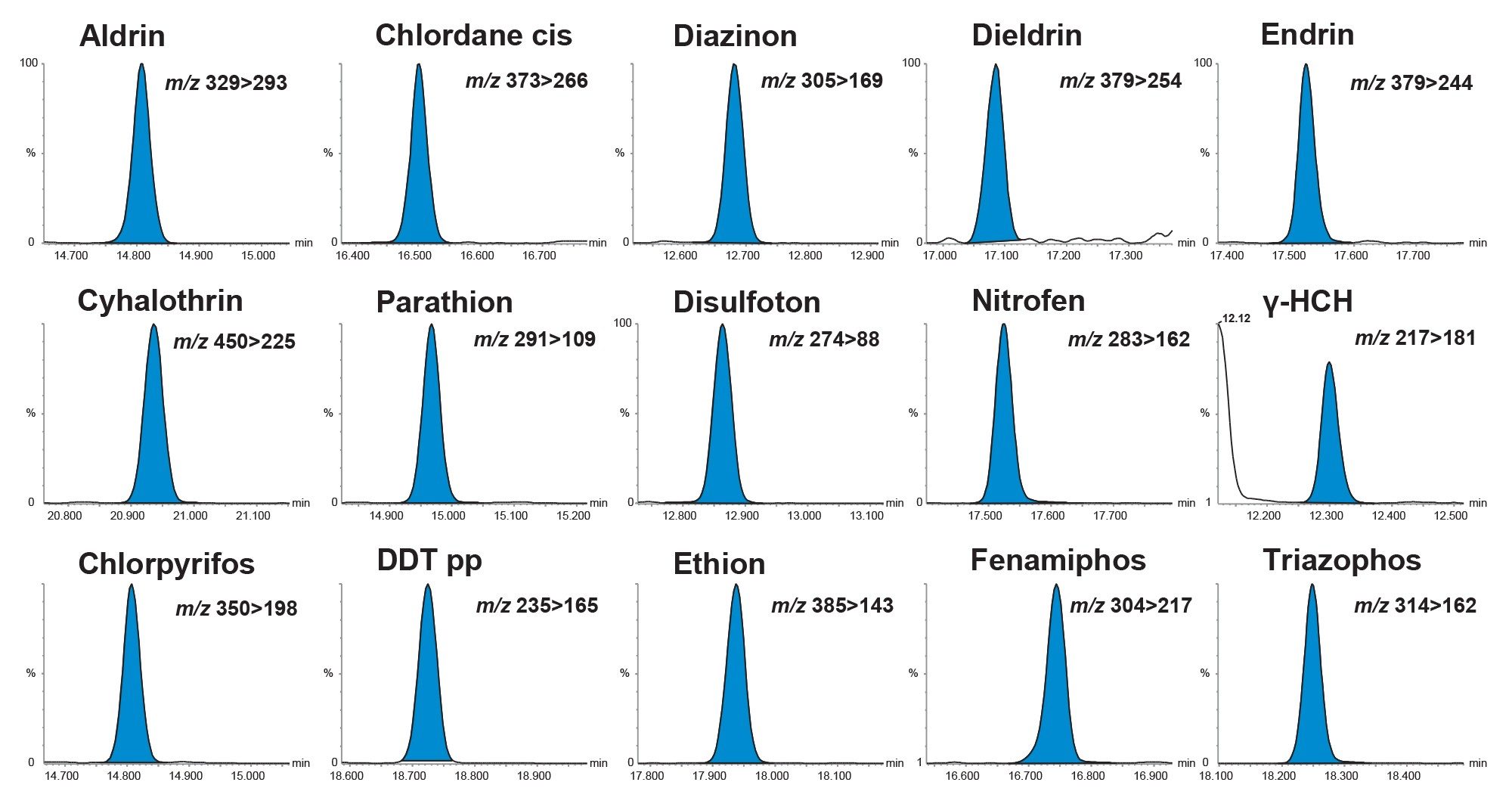
The sensitivity of the method was evaluated by assessment of the response of the matrix-matched standard at the lowest concentration prepared (0.0005 mg/kg) and consideration of the response from the blank. Of the 203 analytes in the method, two were not detected (acequinocyl and aldrin anhydride) and from the remaining all but one could be detected at 0.0005 mg/kg, with 85% of analytes exhibiting responses that indicated that they could be detected at much lower concentrations. Figure 3 show chromatograms from the analysis of a selection of pesticides in the cucumber matrix-matched standard at 0.001 mg/kg. This demonstrates the extreme high sensitivity of the APGC approach with reliable detection for all the analytes at very low concentrations even when the injection volume was 1µL.
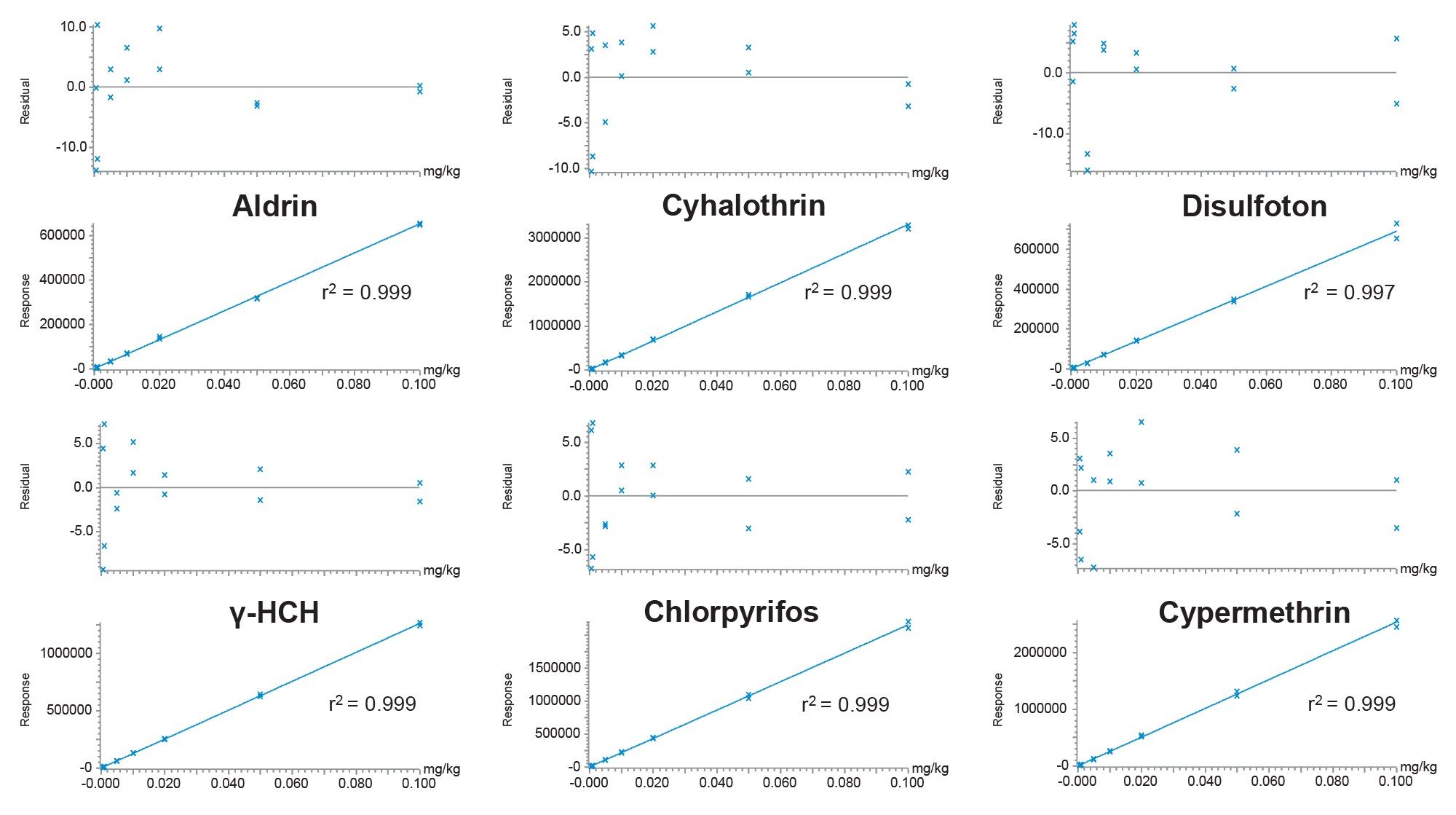
The lowest calibrated level (LCL) for each analyte was established by evaluation of the calibration graph characteristics. The performance for resmethrin was considered semi-quantitative only as the calibration graph exhibited poor residuals (>20%) across the concentration range and a coefficient of determination (r2) of 0.96. Data points at low concentrations were excluded from the calibration graphs of the following analytes due to poor residuals (>20%) and values for LCL were adjusted accordingly: captafol (0.005 mg/kg), chlorothanil (0.005 mg/kg), op DDT (0.005 mg/kg), folpet (0.001 mg/kg) and isodrin (0.001 mg/kg). After these adjustments, all analytes other than resmethrin exhibited residuals well within the ±20% SANTE tolerance.6 Other than captafol (r2=0.98), the graphs for all other analytes had values for r2 >0.99. Bracketed calibration graphs from the analysis of a selection of pesticides in cucumber matrix-matched standards are given in Figure 4.
Identification criteria, retention times and ion ratios, were calculated and flagged using TargetLynx. The retention time and ion ratio of each analyte detected in each spiked sample should correspond to that of the calibration standard reference.6 The retention times of all the analytes were found to be within the tolerance of ±0.1 minute. The ion ratios from the analysis of the samples spiked at 0.001 mg/kg were within ±30% of the average of calibration standards from same sequence for 97% of the analytes, the exceptions being chloroneb, cycloate, diphenylamine, 2, 3, 5, 6-tetrachloroaniline, and tetramethrin. The ion ratios from the data from analysis of the spikes at 0.01 mg/kg were within tolerance for all the analytes.
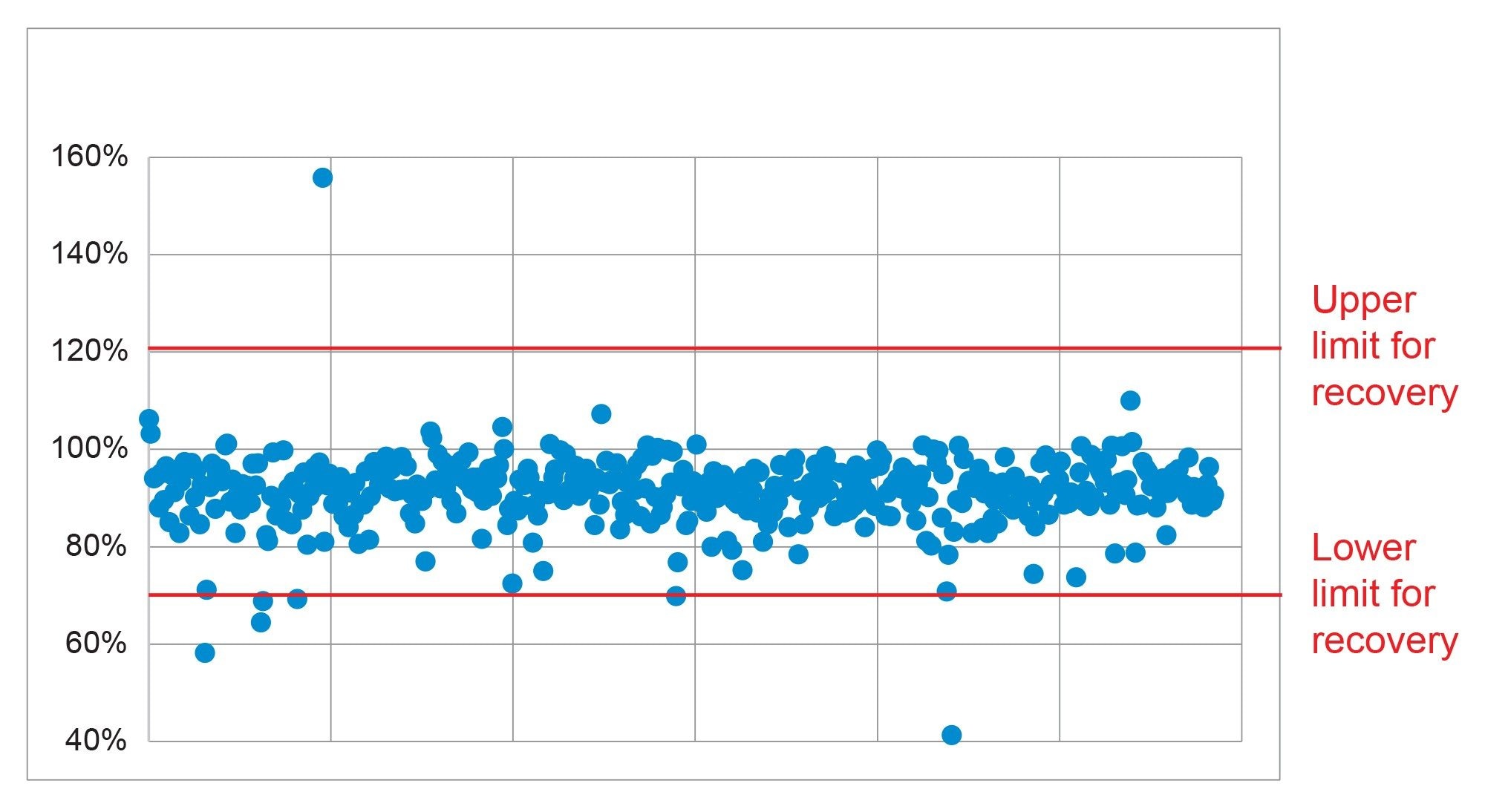
The recovery was evaluated using the data from the analysis of the five replicate spikes, at the two concentrations. The SANTE guidelines specifies an average recovery for each spike level tested to be between 70% and 120%.6 The results from analysis of the spikes at 0.001 mg/kg showed that 94% of the analytes were within that tolerance. The exceptions were azinphos methyl (58%), captafol (64%), chlorfenapyr (69%), chlorothalonil (156%), op DDT (10%), and resmethrin (no results due to poor quantification). At the higher concentration of 0.01 mg/kg, only captafol (69%) was just outside the tolerance, with results for resmethrin (74%) being considered indicative only due to poor calibration. A summary of the recovery results is shown in Figure 5.
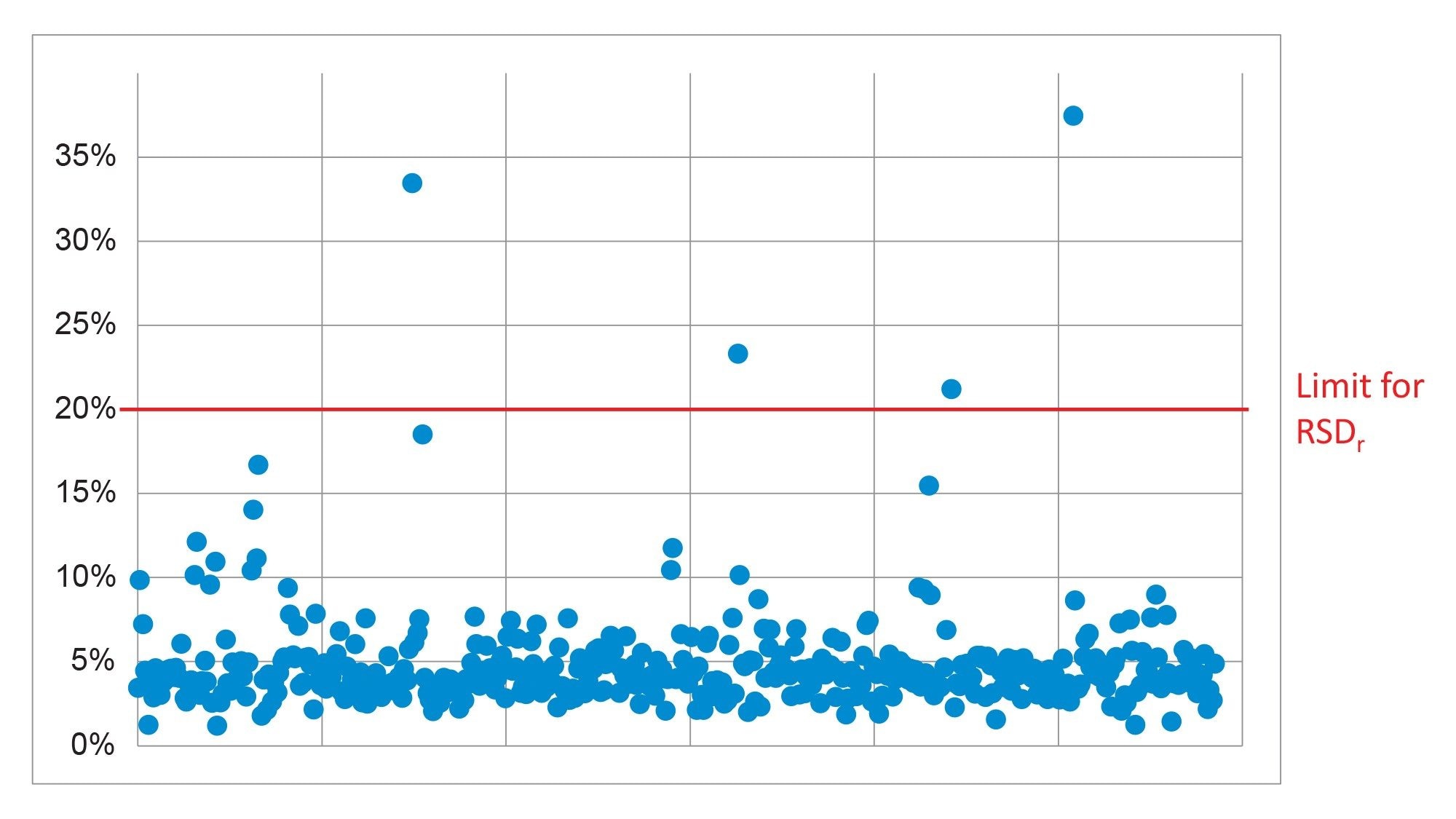
The repeatability (RSDr) of the method was also satisfactory. SANTE guidelines states that RSDr for each spike level tested should be ≤20%.6 At 0.001 mg/kg, 99% of the analytes were within this tolerance. The exceptions were op DDT (34%) and isodrin (23%). At the higher concentration of 0.01 mg/kg, all the analytes exhibited values for RSDr ≤20%. A summary of the repeatability results is shown in Figure 6.
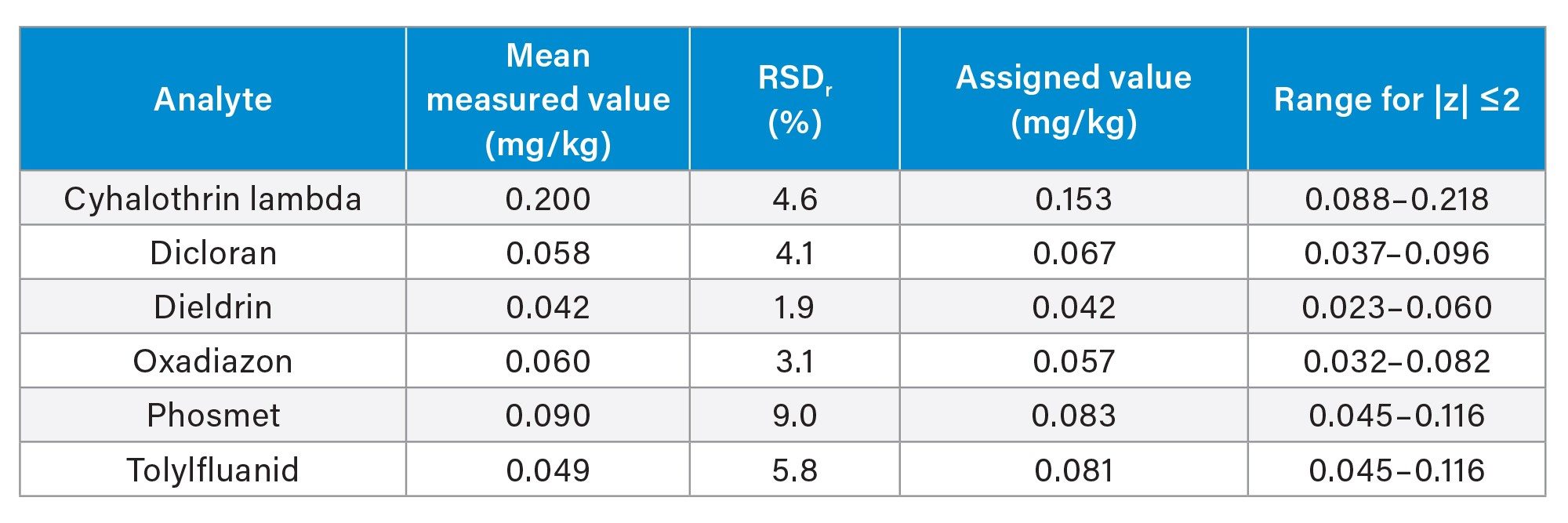
A cucumber puree reference material (T19290QC) was analyzed to further evaluate the performance of the method. The measured values agreed well with the assigned values for five of the analytes, with good repeatability (Table 1). The mean value for tolylfluanid (0.049 mg/kg) was much lower than the assigned value of (0.081 mg/kg) but inside the concentration range within the limits of ±2 z-scores (0.040–0.103 mg/kg). These measurements exhibited good repeatability (RSD 5.8%). Tolyfluanid is associated with stability issues and has been shown to degrade rapidly during sample preparation, if samples are allowed to defrost and during dSPE clean-up when using the PSA sorbent.7,8
Conclusion
This application note describes a sensitive and accurate multiresidue method for the determination of pesticide residues using GC-MS/MS (Xevo TQ-XS fitted with APGC). The method allowed for reliable quantitation down to concentrations well below typical MRLs and was successfully validated according the SANTE guidelines, presenting satisfactory results for 200 pesticides in cucumber. The method exhibited very high sensitivity (LODs typically <0.0005 mg/kg) without the need for solvent exchange, PTV or large volume injection. The results from analysis of the spikes showed almost all the analytes were within the required tolerance for recovery and repeatability, respectively. For example, at 0.001 mg/kg, 94% were within the tolerance for recovery and 99% for repeatability. The procedure can be applied to the analysis of other commodities after suitable validation. This method has been demonstrated as suitable for checking compliance with MRLs and has the potential for determination at much lower concentrations.
References
- Niu Y et al. Atmospheric Pressure Chemical Ionization Source as an Advantageous Technique for Gas Chromatography-Tandem Mass Spectrometry. Trends Anal. Chem. (2020) 132:116053.
- Cherta L et al. Application of Gas Chromatography–(Triple Quadrupole) Mass Spectrometry With Atmospheric Pressure Chemical Ionization for the Determination of Multiclass Pesticides in Fruits and Vegetables. J Chromatogr. A (2013) 1314:224–240.
- Saito-Shida S et al. Quantitative Analysis of Pesticide Residues in Tea by Gas Chromatography–Tandem Mass Spectrometry With Atmospheric Pressure Chemical Ionization. J Chromatogr. B (2020a) 1143:122057.
- Saito-Shida S et al. Multi-Residue Determination of Pesticides in Green Tea by Gas Chromatography-Tandem Mass Spectrometry With Atmospheric Pressure Chemical Ionisation Using Nitrogen as the Carrier Gas. Food Addit. Contam. Part A (2020b) 38(1): 125–135.
- European Committee for Standardisation (CEN) EN 15662:2018. Foods of Plant Origin - Multimethod for the Determination of Pesticide Residues Using Gc- And LC- Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive Spe - Modular Quechers-Method.
- Document No. SANTE/12682/2019. Guidance Document on Analytical Quality, Control, and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. 2019.
- Fussell R et al. Assessment of the Stability of Pesticides during Cryogenic Sample Processing. 1. Apples. Agric. Food Chem. (2002) 50(3):441–448.
- Rutkowska E et al. Modification of Multiresidue QuEChERS Protocol to Minimize Matrix Effect and Improve Recoveries for Determination of Pesticide Residues in Dried Herbs Followed by GC-MS/MS. Food Anal. Methods (2018) 11:709–724.
Annex
720007654, June 2022