
This is an Application Brief and does not contain a detailed Experimental section.
This application brief describes Waters 2420 Waters 2420 Evaporative Light Scattering Detector.
Evaporative Light Scattering detection (ELSD) is a HPLC detection technique based on the ability of particles to scatter light when they pass through a beam of light. The detector will respond to compounds that are less volatile than the mobile phase. Evaporative light scattering therefore offers an alternative detection strategy for compounds that do not have a UV chromophore, and, unlike refractive index detectors, ELSD is compatible with gradient analyses. Evaporative light scattering detection is compatible with a wide range of flow rates and mobile phase compositions and can be part of a multi-detection scheme (for example: PDA/MS/ELSD).
Evaporative Light scattering detection is useful for wide range of compounds and compound classes including:

An ELSD has three basic elements: nebulizer, desolvation tube (often referred to as a drift tube) and scattering chamber. In summary, a solvent stream is nebulized and the droplets formed in the nebulizer are entrained in a flow of gas. The droplets are evaporated in the desolvation region and, if there was non-volatile analyte present in the solvent stream, dry particles remain which are carried along in the flowing gas and solvent vapor stream. A beam of light intersects the path of the flowing stream. If dry particles are present, they scatter light. The scattered light is measured and the intensity of this light is a function of the size and number of such particles.
Column effluent is passed through a narrow needle and mixed with gas (typically nitrogen, but any dry, particulate free gas can be used) to produce an aerosol of droplets. The 2420 nebulizers are tailored to flow rate, a high flow nebulizer for flow rates of 0.30–3.0 mL/minute, and a low flow nebulizer for flow rates of 0.05–0.50 mL/minute. Nebulizer gas flow rate can be adjusted to optimize detector signal and minimize noise. High gas flows give smaller droplets that require less heat to evaporate off the solvent to leave the non-volatile sample. Lower gas flows give large droplets (which generally generate larger particles, giving larger signals), but require more heat to evaporate the solvent, leaving the non-volatile sample and can, if the solvent is not fully evaporated away, cause noise. Droplets that are too large to pass into the drift tube are removed via a siphon tube to waste. The nebulizer region can be heated to improve evaporation and increase signal; this temperature setting should be determined experimentally.
Nebulized droplets are driven into the drift tube by the carrier gas and diffuse down the drift tube. Once in the drift tube, particles are generated by the evaporation of the mobile phase components leaving the non-volatile portion of eluent as particles. Ideally, the drift tube is heated to a temperature that facilitates solvent removal without sample depletion. Higher drift tube temperatures are more efficient at removing solvent, but may evaporate semi-volatile sample components leading to a reduction in signal. Optimal drift tube temperatures are best determined experimentally for each individual compound of interest. When dealing with totally unknown sample components, users should use the lowest drift tube temperature that adequately evaporates the mobile phase at its given flow rate.
The scattering chamber is the equivalent of the flow cell in UV/Visible detectors. Incident light is generated from a quartz-halogen lamp and passes through a series of lenses and mirrors into the scattering chamber where it interacts with the sample particles. A photomultiplier tube detects light scattered by particles and the resulting signal is output to a data collection device (directly to Empower or MassLynx Software, or other software through a SAT/IN module). The optics bench is also heated to avoid condensation of evaporated mobile phase components. After detection, the particle stream and evaporated solvent are exhausted from the detector.
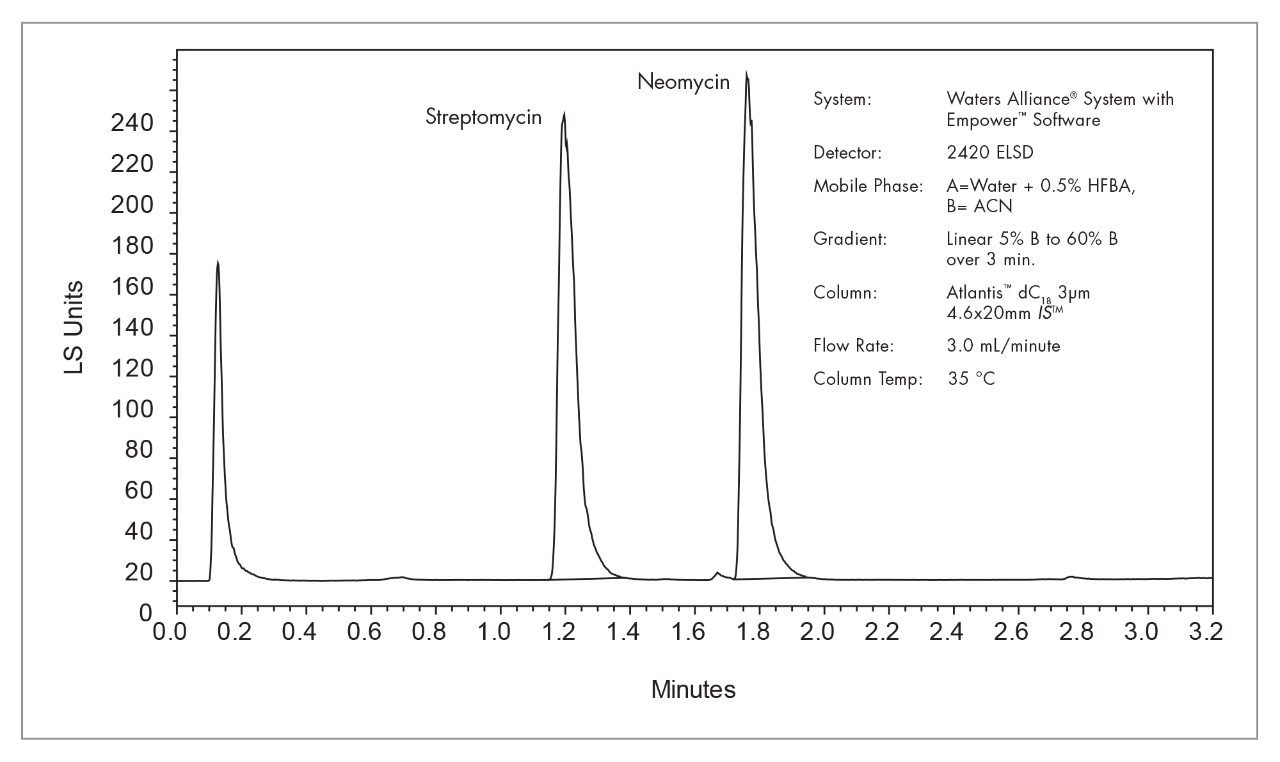
|
System: |
Waters Alliance System with Empower Software |
|
Detector: |
2420 ELSD |
|
Column: |
Symmetry C18 Column 5 μm 3.9 x 150 mm |
|
Mobil phase: |
A=Water + 1% Formic Acid, B= Methanol |
|
Composition: |
Isocratic 36% A, 64% B |
|
Flow Rate: |
1.0 mL/minute |
|
Column temp.: |
30 °C |
ELSD’s are not spectrometric detectors; therefore, they do not obey Beer’s Law and are fundamentally non-linear. Scattering is independent of the particle’s chemical properties. It is a function of multiple processes including Rayleigh scattering, Mei scattering, refraction, and reflection. The size and shape of the particle, number of particles, and the wavelength of the incident light all impact light scattering. The absolute detector response is a mixture of all scattering types, although any one type may predominate in a given sample. Calibration curves produced by an ELSD best fit to quadratic equations, but may also be fit to log/log curves.
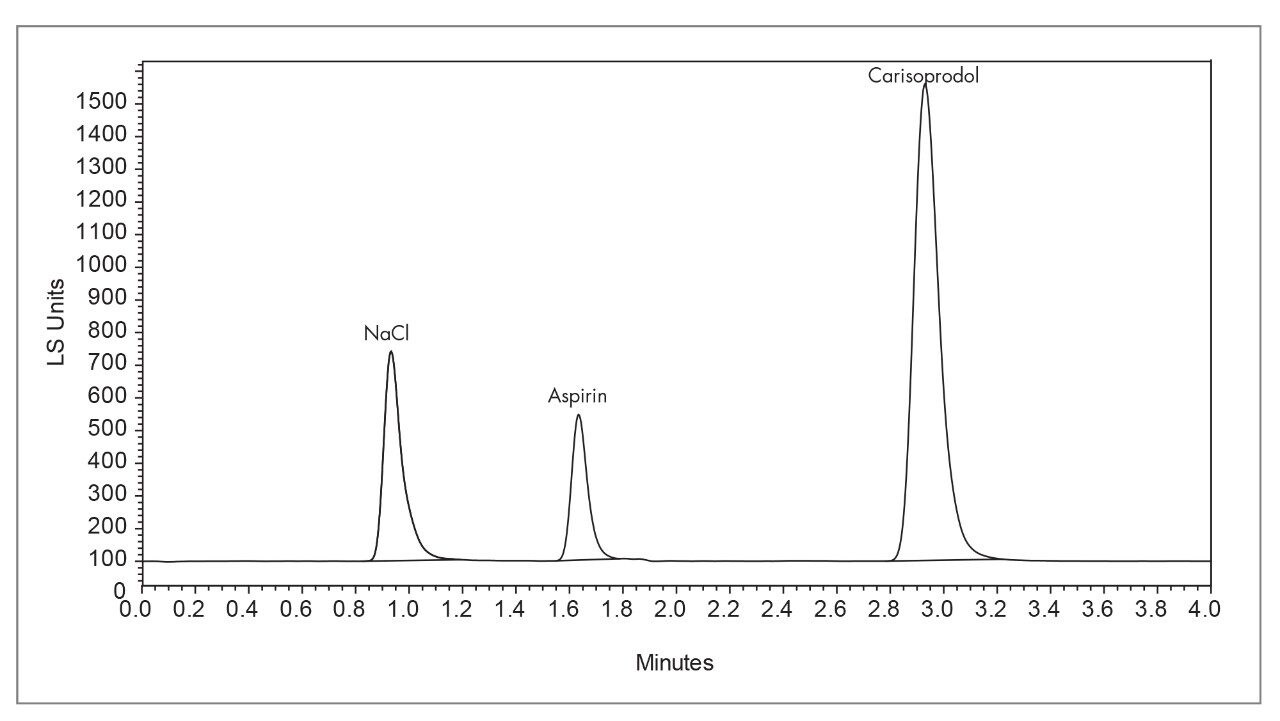
|
System: |
Waters Alliance System with Empower Software |
|
Detector: |
2420 ELSD |
|
Column: |
Atlantis dC18 5 μm 4.6 x 250 mm |
|
Mobil phase: |
A=Water + 0.1% TFA, B= ACN + 0.1% TFA |
|
Gradient: |
5 min Isocratic hold, Linear 0% B to 30% B over next 30 min. |
|
Flow Rate: |
1.0 mL/minute |
|
Column temp.: |
20 °C |
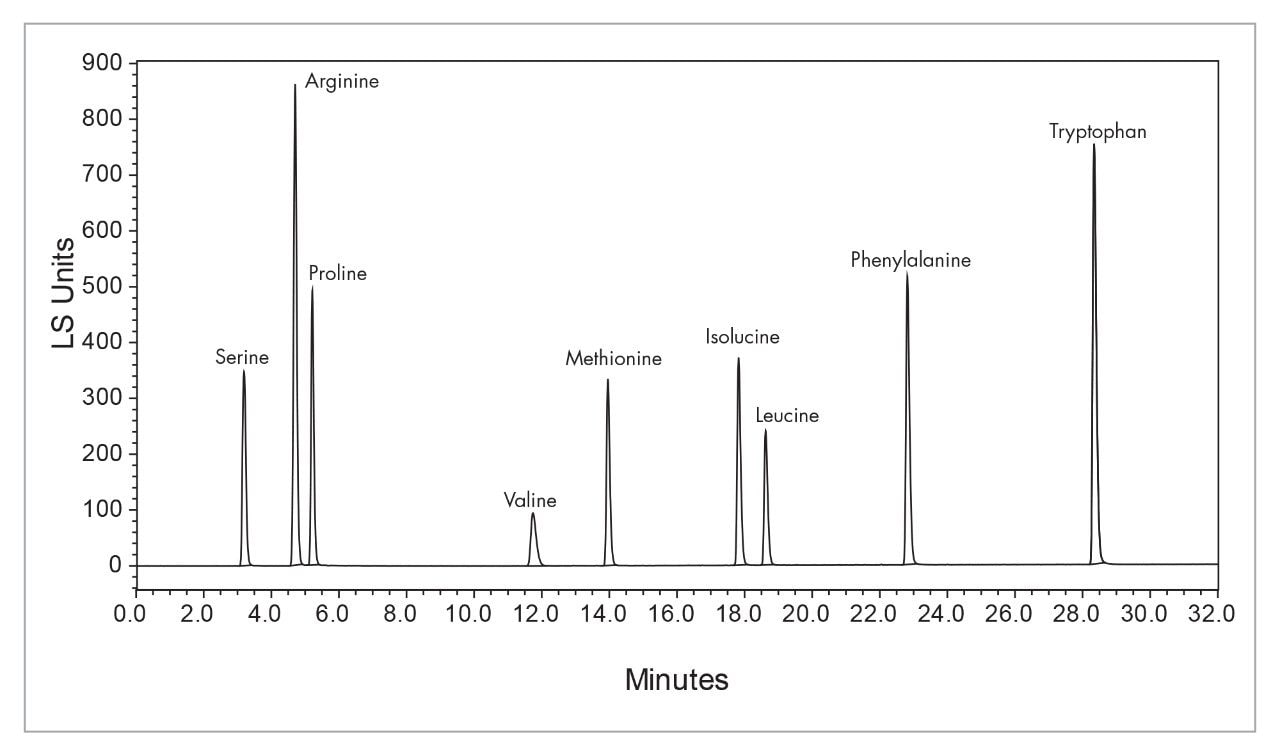
|
System: |
Waters Alliance System with Empower Software |
|
Detector: |
2420 ELSD |
|
Column: |
Atlantis dC18 3 μm 4.6 x 20 mm IS |
|
Mobil phase: |
A=Water B= Methanol |
|
Gradient: |
Linear 5% B to 30% B over 2 min. |
|
Flow Rate: |
3.0 mL/minute |
|
Column temp.: |
40 °C |
720000804, January 2004