ACQUITY UPLC with Ink-Jet Inks (Part 2): UV and MS Detection
ACQUITY UPLC with Ink-Jet Inks (Part 2): UV and MS Detection

This application note describes the comparison of ink-jet formulas from generic and brand printer cartridges utilizing the Waters ACQUITY UPLC/UV System coupled with the Waters ZQ mass detector.
Information-rich, time-efficient tool for identifying and differentiating the dyes and polymeric components in ink-jet ink cartridges
Ink-jet printing applies not only to office documents but also to such diverse applications as kayaks, beverage cans, cardboard cartons and textiles. Ink-jet inks are complex mixtures and those that use organic dyes can also contain water, organic solvents, bactericides/ fungicides, binders, and other additives depending on the end use. It is essential that ink-jet inks are formulated for the system or process and surface to be printed. High contrast colors, lightfastness, and good adhesion properties are typical requirements for ink.
This application note describes the comparison of ink-jet formulas from generic and brand printer cartridges utilizing the Waters ACQUITY UPLC/UV System coupled with the Waters ZQ Mass Detector. The ink dyes and the polymeric surfactant ingredients were separated and detected in two minutes using the ACQUITY UPLC System. This is 10 times faster than traditional RP-HPLC (reversed phase), which resolves dyes1-4 or polymers5 of similar chemical structures in approximately 20 minutes.
The resolution, sensitivity and speed enhancements from UPLC combined with UV and MS detection represents a powerful method for analyzing ink dyes and evaluating the photo-stability of dye components.6-9 The focus of the previous application note in this series9 was on the ACQUITY UPLC System with UV detection only. This note describes a 2-minute method to separate ink-jet dyes and polymer ingredients using the UPLC/UV/ZQ single quadrupole MS system.
The ability to quickly and unambiguously characterize ink formulas can facilitate workflow in production such as certifying lot-to-lot variation and product troubleshooting. Central analytical and research labs will find this approach useful for evaluating competitive products and in patent infringement defense scenarios.
The ink-jet solutions (0.5–1 μL) were extracted from ink-jet printer cartridges by pipette and diluted with 1–2 mL of D.I. water. The sample solutions were filtered with 25 mm GHP Acrodiscs filters (WAT200514) and placed in Max Recovery vials (186000327c) for UPLC analysis.
|
System: |
ACQUITY UPLC with ACQUITY UV detector and Waters ZQ 2000 |
|
Software: |
Empower Software |
|
Column: |
ACQUITY UPLC BEH C18 2.1 x 50 mm |
|
Column temp.: |
50 °C |
|
Weak wash: |
95:5 Water:CH3CN (500 μL) |
|
Strong wash: |
50:50 Water:CH3CN (300 μL) |
|
Seal wash: |
90:10 Water:CH3CN (5 min) |
|
Mobile phase A: |
10 mM NH4HCO3, pH 9.8 |
|
Mobile phase B: |
CH3CN |
|
Linear Gradient: |
10% B to 65% B in two minutes |
|
Flow rate: |
1 mL/min |
|
Injection: |
5 μL |
|
Detection: |
UV absorbance (at 400 nm) |
|
Sampling rate: |
20 pts/s |
|
Filter response: |
0.1 s |
|
Probe: |
ES+ |
|
Cone (V): |
40 |
|
RF Lens (V): |
0.5 |
|
Probe: |
ES- |
|
Cone (V): |
-30 |
|
RF lens (V): |
-0.5 |
|
Desolvation temp. (°C): |
450 |
|
Desolvation gas (L/Hr): |
800 |
|
HM resolution: |
15 |
|
Multiplier for ES-: |
650 |
|
Scan time: |
0.22s |
|
ES capillary (kV): |
3.2 |
|
Extractor (V): |
2 |
|
Multiplier for ES+: |
500 |
|
ES capillary (kV): |
-3 |
|
Extractor (V): |
-2 |
|
Source Temp (°C): |
140 |
|
Cone Gas flow (L/Hr): |
50 |
|
LM Resolution: |
15 |
|
Ion Energy: |
0.3 |
|
Scan Range: |
150 to 1000 Da |
|
Inter-scan delay: |
0.05s |
Yellow ink-jet inks from three different vendors (brand and generic) were separated using the ACQUITY UPLC system with a two-minute linear gradient method and ammonium bicarbonate pH 9.8 and acetonitrile as the mobile phases. The ZQ single quadrupole mass detector combined with UV detection were used for peak tracking and identification of dye components. Figures 1, 2, and 3 show the UV absorbance, negative and positive ES TIC (electrospray total ion current) chromatograms of the ink-jet inks.
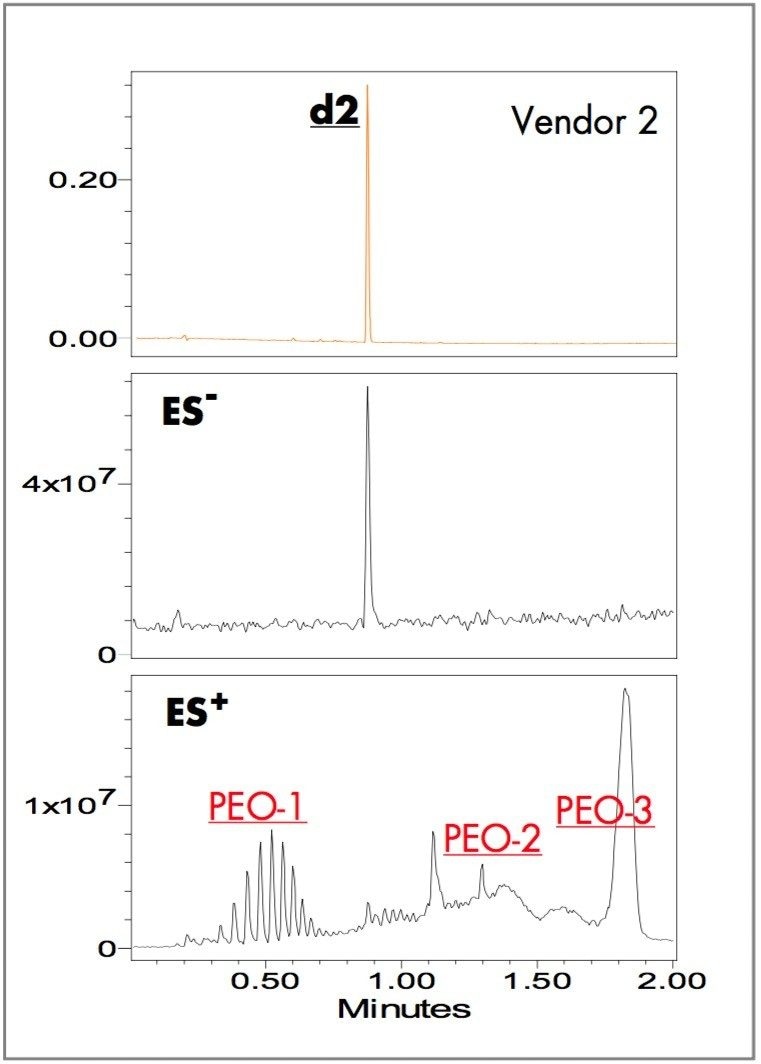
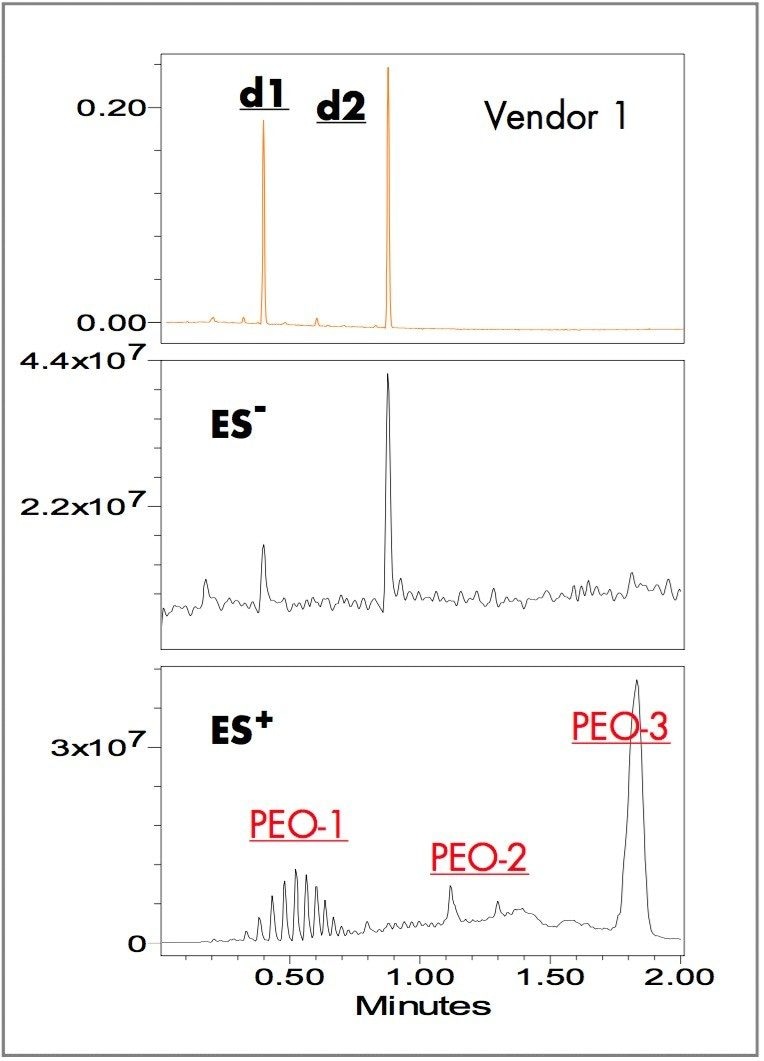
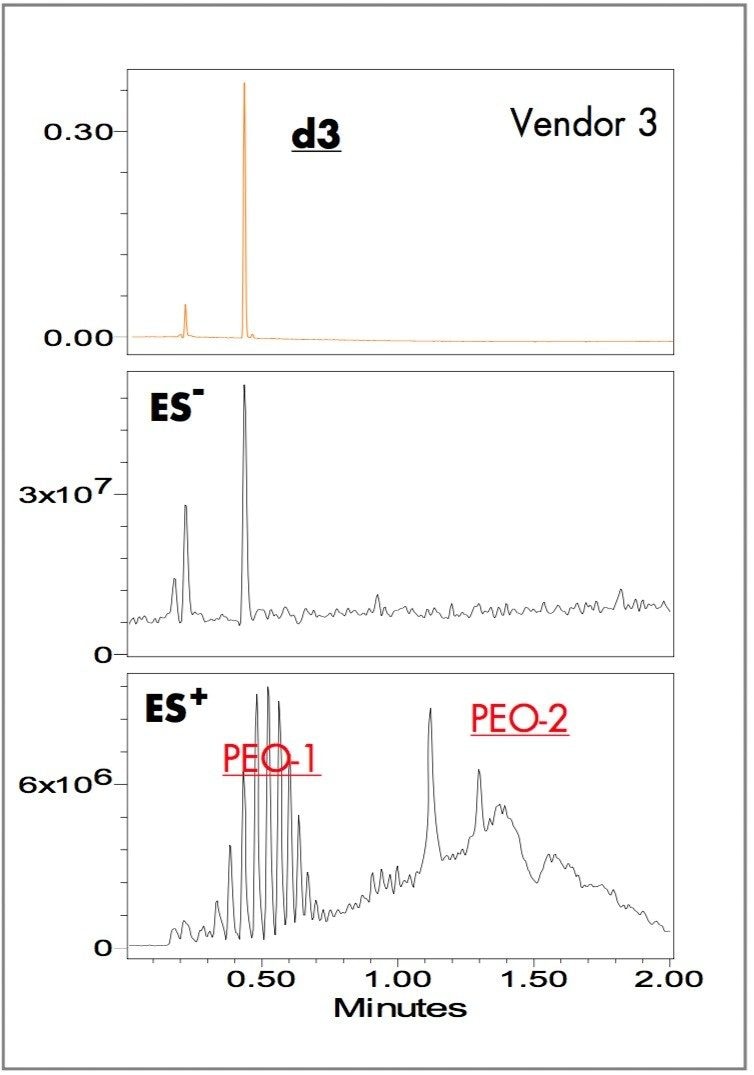
The UV chromatograms show two major dye components in the Vendor 1 ink at 0.40 (d1) and 0.88 (d2) minutes; one major dye component in the Vendor 2 ink at 0.88 (d2) minutes; one major dye component in the Vendor 3 ink at 0.43 (d3) minutes. The ES- TIC chromatograms matched the peaks observed with the UV detector. The ES+ TIC chromatograms showed peaks not observed in either the UV or the ES- TIC chromatograms illustrating the value of combining UV detection with positive/negative ion MS.
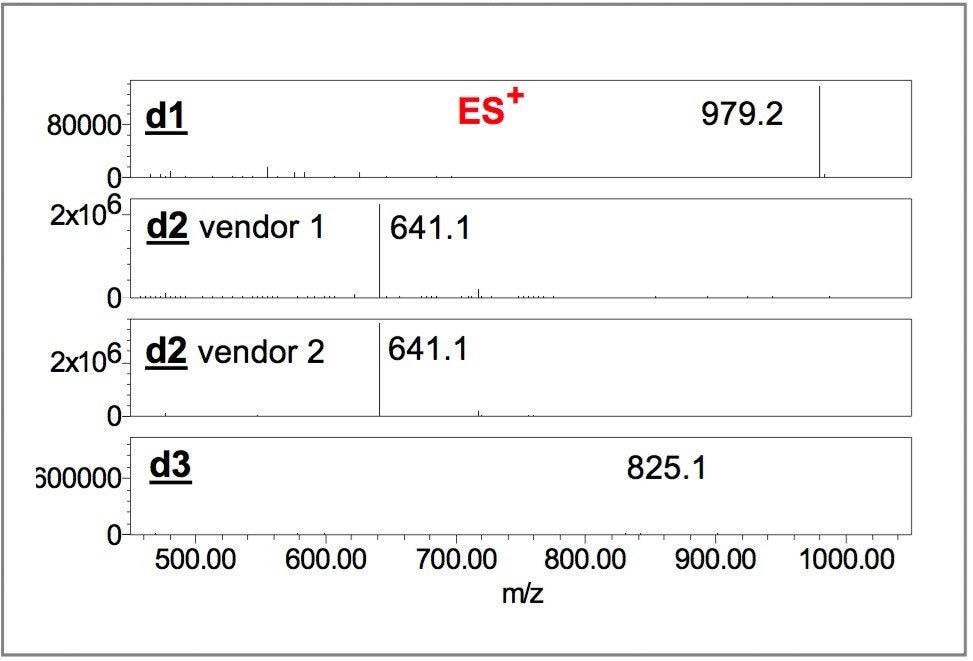
Figures 4 and 5 show negative and positive ion mass spectra of dye components d1, d2 and d3. The mass spectrometry data enabled the unambiguous differentiation among Vendors 1, 2 and 3.
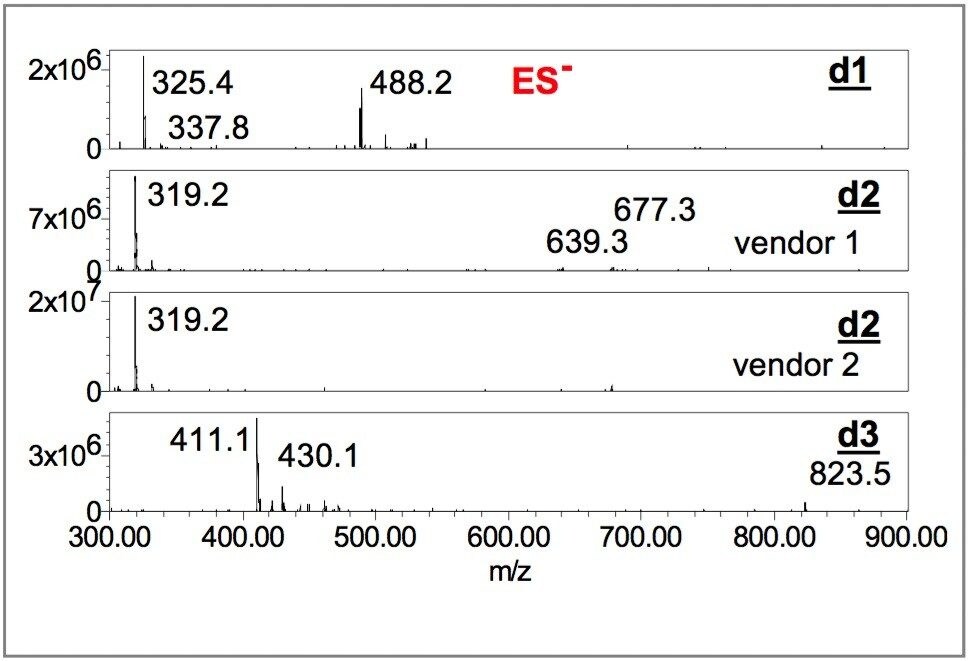
The interpretation of negative-ion mass spectra of acidic ink-jet dyes is straightforward.4,10 The molecular mass of the acidic dye, M, can be determined from the series of [M-nH]n- ions, where n is the number of charges carried by the ion and H is hydrogen. For salt adducts [M- (n+x)H+xS]n-, where the maximum value of n or (n+x) is equal to the total number of acidic groups, S is the atomic weight of adduct species (23 for Na, 39 for K, etc.).
Although the positive-ion peak intensity of ink dyes is weaker than the negative-ion intensity, mass spectra can be extracted from the ES+ TIC chromatograms. The m/z ratios of positive ions are useful for further confirming the molecular mass of the dye calculated using the m/z ratios of a series of negative ions.
The molecular mass of d1, 978 Da, is calculated from the m/z of negative ions 325 ([M-3H]3-), and 488 ([M-2H]2-) ;10 and confirmed by the m/z of potassium adduct 337.8 ([M-4H+K]3-), and the m/z of M+H ion at 979 ([M+H]1+). The molecular mass of d2, with 640 Da is obtained from m/z of 319 ([M-2H]2-), and m/z 639 ([MH] 1-);10 and verified by the m/z of potassium adduct 677 ([M-2H+K]1-), and the m/z of M+H ion at 641 ([M+H]1+). The molecular mass of d3 with 824 Da, is determined from the m/z of 411 ([M-2H]2-) and, 823 ([M-H]1-);10 and validated by the m/z of potassium adduct 430 ([M-3H+K]2-), and the m/z of M+H ion at 825 ([M+H]1+).
Although d1 and d3 have similar retention times, 0.40 and 0.43 minutes, respectively, the MS data clearly indicate different species. The mass spectra of ES negative and positive ions unambiguously confirm the presence of d2 in the formulas of both Vendors 1 and 2.
The inks of Vendors 1 and 3 have no common dye components. These results indicate the utility of applying the UPLC/UV/ZQ system in dye related industries for fast screening of imitation products, competitive analysis and patent infringement.
The ES positive-ion mass spectra of PEO-1, PEO-2, and PEO-3 consist of several adjacent ion envelopes each based on 44 Da spacing. This indicates poly(ethylene oxide) based polymers with different molecular weight distributions, end groups, and branches.
The positive-ion TIC chromatograms of Vendors 1 and 2 have nearly identical peaks (Figures1 and 2). A notable difference is that the relative peak intensities in PEO-3 in the Vendor 1 ink are larger than those in the Vendor 2 ink. Vendor 3 contains all the peak components of Vendors 1 and 2 except PEO-3 (Figure 3).
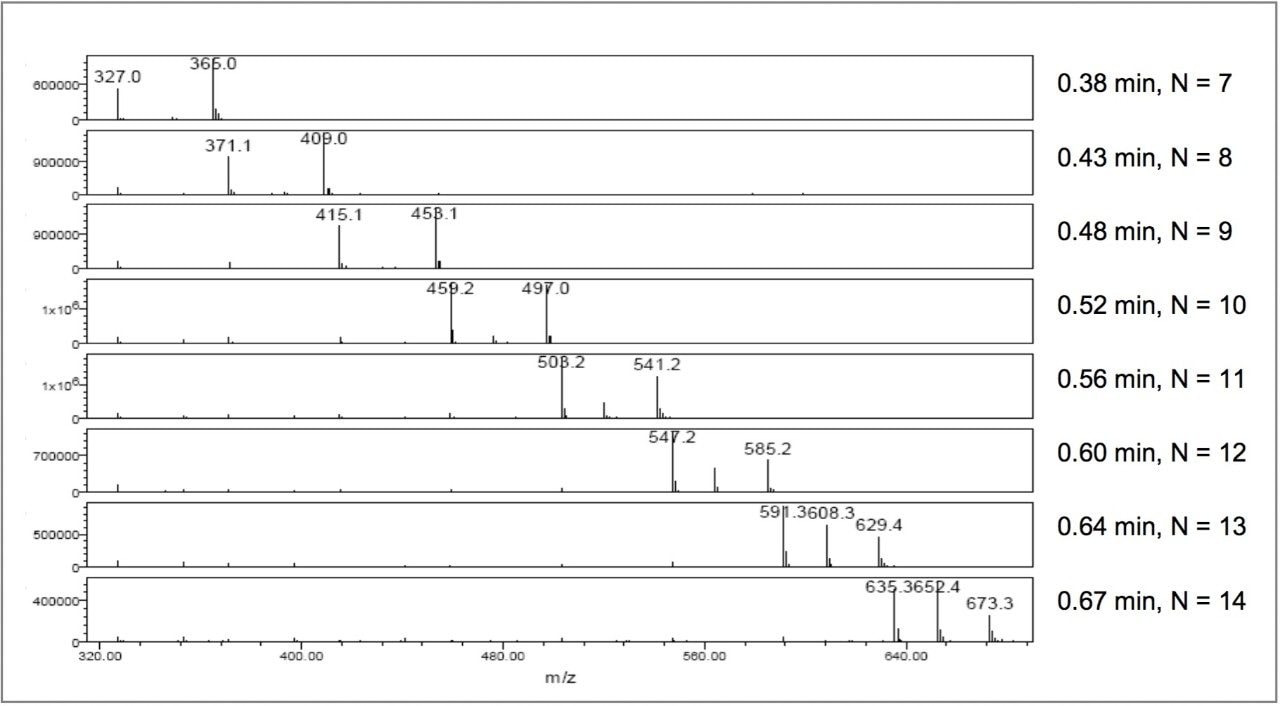
Figure 6 shows the extracted mass spectra of eight PEO-1 peaks with retention times from 0.38 to 0.67 minutes. The mass spectra of each peak contained two or three major ions that are recognized as [H(OCH2CH2)NOH+H]+ ions accompanied by salt adducts, [H(OCH2CH2)NOH+NH4]+, or [H(OCH2CH2)NOH+K]+, where N is 7 to 14. For example, the mass spectrum of the peak at 0.67 minutes has ions at m/z 635.3, 652.3, and 673.3 (Da), which are consistent with [H(OCH2CH2)14OH+H]+, [H(OCH2CH2)14OH+NH4]+, and [H(OCH2CH2)14OH+K]+.
These results for the separation of polymers with the UPLC/UV/ZQ system are 10 times faster than similar results reported with conventional RP-HPLC and provide molecular weight and polymer composition information.5
The Waters ACQUITY UPLC combined with UV and ZQ MS detectors provides an information-rich, time-efficient tool for identifying and differentiating the dyes and polymeric components in ink-jet ink cartridges.
There are multiple benefits in combining MS and UV detection after UPLC separation for ink-jet ink analyses. The retention time of UV (400 nm) chromatogram peaks identifies the dye components; whereas, the extracted mass spectra of negative-ion TIC chromatograms reveal the molecular weight of dyes and the extracted mass spectra of positive-ion chromatograms reveal polymer structures that were undetected by UV.
Potential applications are competitive deformulation, imitation product verification and patent infringement litigation, batch-to-batch product quality control and troubleshooting. The resolution power and separation speed for polymers and dyes will prove useful for ink-jet ink developers and producers.
To calculate molecular mass of d1 by using equation 2. we may define the following:
m1 = 488; m2 = 325, and n1 is given by (325 + 1)/(488 - 325) = 2
By using equation 1, M of d1 is given by 488 x 2 + 2 = 978.
To calculate molecular mass of d2 by using equation 2. we may define the following:
m1 = 639; m2 = 319, and n1 is given by (319 + 1)/(639 - 319) = 1
By using equation 1, M of d2 is given by 639 x 1 + 1 = 640.
To calculate molecular mass of d3 by using equation 2. we may define the following:
m1 = 823; m2 = 411, and n1 is given by (411 + 1)/(823 - 411) = 1
By using equation 1, M of d3 is given by 823 x 1 + 1 = 824.
720001463, January 2006