In the following application, we will present the impact that mobile phase composition (pH and ionic strength) can have on this separation. Additionally, we will discuss some of the primary considerations necessary for the successful transfer of a developed SE-UPLC method.
Size-exclusion chromatography (SEC) has been the predominant technique for the analysis of biotherapeutic protein aggregation, or high molecular weight forms (HMW), an important degradation pathway that has been found to correlate with undesired immunogenic effects and/or decreased efficacy.1,2 An additional degradation pathway for monoclonal antibodies (mAb) that can be observed by SEC is non-enzymatic peptide bond hydrolysis in the hinge region of these proteins resulting in antibodies that are missing one or both FAb arms.3 Identification of these low molecular weight degradants (LMW) are provided in a previous Waters Application Note 720004254EN: “Analysis of Proteins by Size-Exclusion Chromatography Coupled with Mass Spectrometry Under Non-Denaturing Conditions”.
The introduction of Ultra Performance Liquid Chromatography (UPLC) or low dispersion systems in combination with sub-2 µm particles has allowed for improvements in these isocratic separations, including higher throughput, improved resolution, and enhanced sensitivity. The advantages of using size-exclusion UPLC (SE-UPLC) for monitoring the extent of mAb hinge region fragmentation in addition to aggregation using a non-denaturing mobile phase have been evaluated for trastuzumab, an anti-HER2 IgG1 mAb. As in any SEC method, a variety of parameters can be adjusted to improve resolution and method robustness. In the following application, we will present the impact that mobile phase composition (pH and ionic strength) can have on this separation. Additionally, we will discuss some of the primary considerations necessary for the successful transfer of a developed SE-UPLC method.
|
Sample Description: |
The IgG1 mAb sample was trastuzumab that was analyzed past expiry (21 mg/mL). |
|
System: |
ACQUITY UPLC H-Class Bio System with TUV and Titanium Flow Cell |
|
Wavelength: |
280 nm |
|
Column: |
ACQUITY BEH200 SEC 1.7 μm, 4.6 x 300 mm (p/n 186005226) |
|
Column Temp.: |
25 °C (without Active Pre-Heater Assembly) |
|
Sample Temp.: |
4 °C |
|
Injection Volume: |
5 μL (unless specified otherwise) |
|
Flow Rate: |
0.4 mL/min |
|
Mobile Phase: |
Prepared using Auto•Blend Plus Technology four solutions: A: 100 mM sodium phosphate monobasic B: 100 mM sodium phosphate dibasic C: 1.0 M sodium chloride D: water |
|
Final Target Composition: |
25 mM sodium phosphate, pH 6.8, 200 mM sodium Chloride, (unless specified otherwise) |
|
Software: |
Empower 2 with Auto•Blend Plus |
In the following discussion, we will outline some of the parameters that should be evaluated when developing an SE-UPLC method. While these SEC method development steps are demonstrated on UPLC SEC, the same principles apply to any SEC separation. Method performance will be evaluated based on peak shape, resolution, and quantitative reliability.
Following the selection of a column with appropriate characteristics such as pore size, particle composition and bonding, particle size, and column geometry, the next step in developing a SEC method typically involves evaluation of the mobile phase to ensure good peak shape and component resolution. Modifying the ionic strength and pH of the mobile phase can be easily accomplished with a quaternary solvent management system in combination with software that can take advantage of this four-solvent blending system.4 This approach was used throughout the studies described.
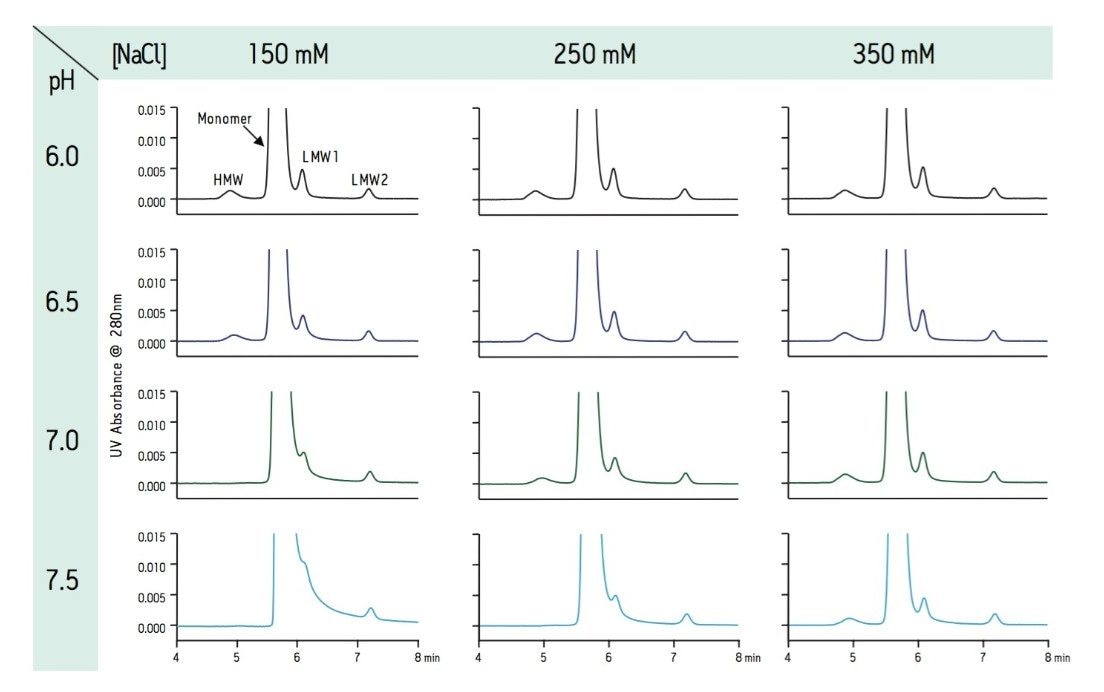
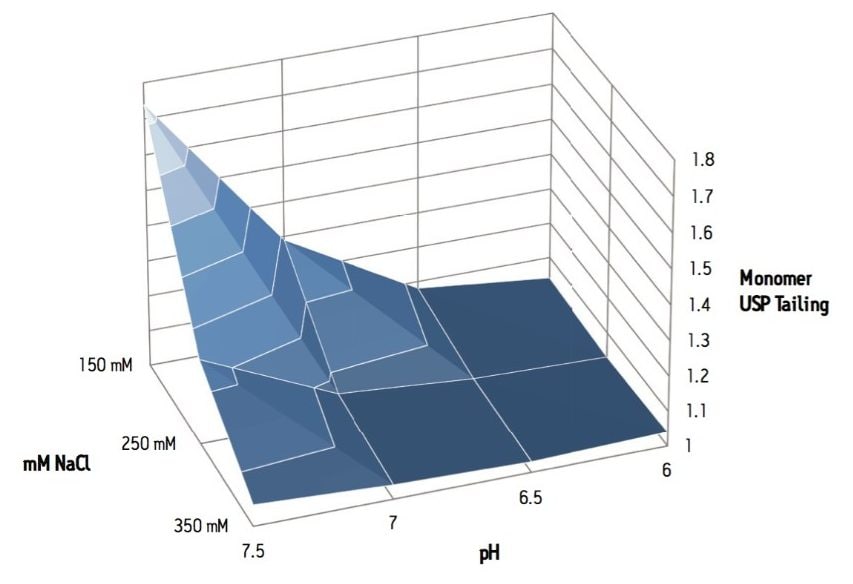
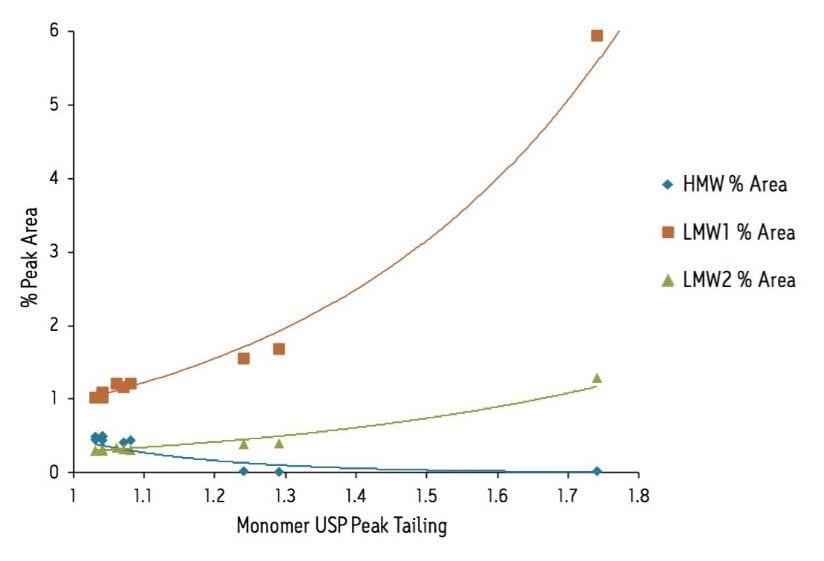
The SE-UPLC separation of trastuzumab was evaluated at NaCl concentrations between 150 and 350 mM and pH levels between 6.0 and 7.5 (Figure 1). In this design space and based on a visual assessment the chromatographic profile appears to be optimal and stable at NaCl levels of 250 mM through and 350 mM, and at a pH of 6.0 to 6.5 (25 mM phosphate buffer). As is commonly observed with gel filtration packing materials, lower ionic strength mobile phases lead to increased peak tailing for the monomeric mAb4. This observed peak tailing effect was also less pronounced at pH values 6.0 and 6.5 (Figure 2), which in this case closely matches the pH of the trastuzumab formulation (pH 6.0). Pragmatically, the results of interest for this analysis are the integrated percent peak areas for the product-related impurities, the HMW and LMW forms. Within the studied mobile phase compositions, in which the HMW and the later eluting LMW2 (FAb arm) peaks are observed, the integrated percentage of these impurities are not significantly influenced by the peak shape (tailing factor) of the intact mAb monomer. However, increases in peak tailing of the monomer can result in significant increases in the integrated percent area of the earlier eluting LMW1 impurity (mAb minus one FAb arm) peak (Figure 3). Based on these data, an appropriate mobile phase that should result in reproducible chromatographic profiles would be at pH 6.25 (25 mM phosphate buffer) with 300 mM NaCl (Figure 4).
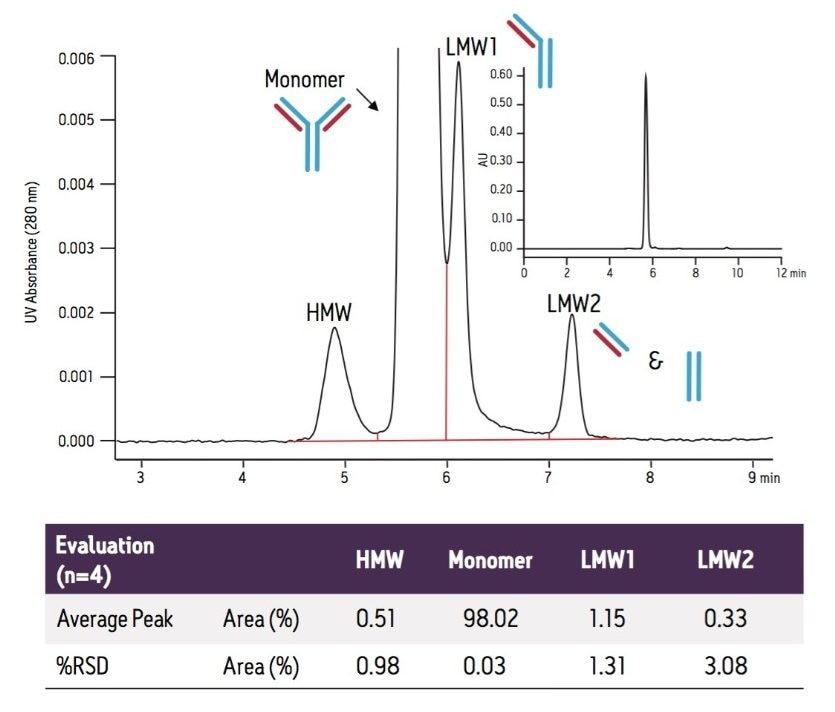
It should be noted that while this approach can provide a reproducible assessment of the HMW forms present in a protein sample, the accuracy of the analyses should be assessed by the use of an orthogonal method such as Analytical Ultracentrifugation or Asymmetric Field Flow Fractionation.
Most of the chromatographic separation modes commonly used for the analysis of biotherapeutic proteins rely on a gradient elution of analytes that have been pre-concentrated at the head of the column (e. g. cation exchange and reversed phase). In this format, pre-column dispersion does not significantly degrade chromatographic performance. SEC, however, is operated in an isocratic mode and as a result, in addition to post-column dispersion that is observed for all of these modes, the performance of the SEC separation is far more prone to degradation due to pre-column dispersion. One of the primary advantages of using sub-2 µm particle columns for SEC analysis is the significant decrease in peak dispersion that these columns can provide relative to the larger 5–10 µm particle sizes typically used for SE-HPLC. However, this benefit can be easily obscured by the effects of extra-column dispersion. In this respect, the chromatographic instrumentation used and its configuration play key roles in the dispersion of the analyte peak(s).
With respect to quantitative analysis, the effect of extra-column dispersion is most problematic in SE-UPLC separations in which there are partially resolved analytes. Separations where the peak area of the later eluting analyte of the critical pair is very low in comparison to that of the preceding peak are particularly sensitive to these effects. Once a low-dispersion chromatographic system has been selected and its performance optimized, selection of appropriate tubing and fittings must be made.
For a UV-absorbance based SE-UPLC method, the two system components that contribute to extra-column dispersion are the autosampler and the detector. Low dispersion systems specifically designed to operate at the high pressures needed to realize the full benefits of SE-UPLC are required for this methodology. In this regard, the performance of prospective instrumentation under the expected operating conditions should be evaluated thoroughly.
In addition to the UPLC system, significant extra-column dispersion can also be introduced by the tubing and fittings used to configure the SE-UPLC column into the chromatographic system. The extent of extra-column dispersion contributed by the capillary tubing used in a UPLC system may be estimated by a transition equation that was derived from the Taylor-Aris expression, which defines dispersion in long tubes and the equation defined by Attwood and Golay for dispersion in short tubes:
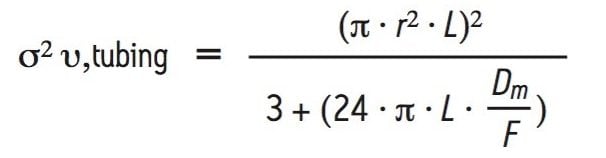
where σ2 υ,tubing is the variance or band spreading contribution of the tubing, r and L are the radius and length of the capillary σ2 υ, tubing is the diffusion coefficient of the analyte, and F is the flow rate5. However, for trastuzumab, which has a diffusion coefficient of approximately 4 × 10-7 cm2/s,6 and at the suggested operating flow rate of 0.4 mL/min, this equation may be simplified back to the Attwood-Golay expression for the limiting case of no diffusion of the analyte until tubing lengths begin to exceed several meters.
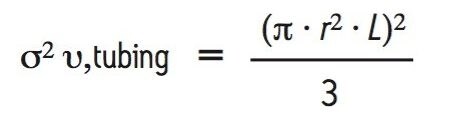
The above expression shows that the contribution to peak dispersion due to the tubing used to configure a UPLC system is proportional to the length of the tubing raised to the second power and to the inner-diameter raised to the fourth-power. To the chromatographer, this relationship suggests that SE-UPLC performance can be maximized by configuring a system with tubing having the shortest length and the smallest diameters insofar as is practical.
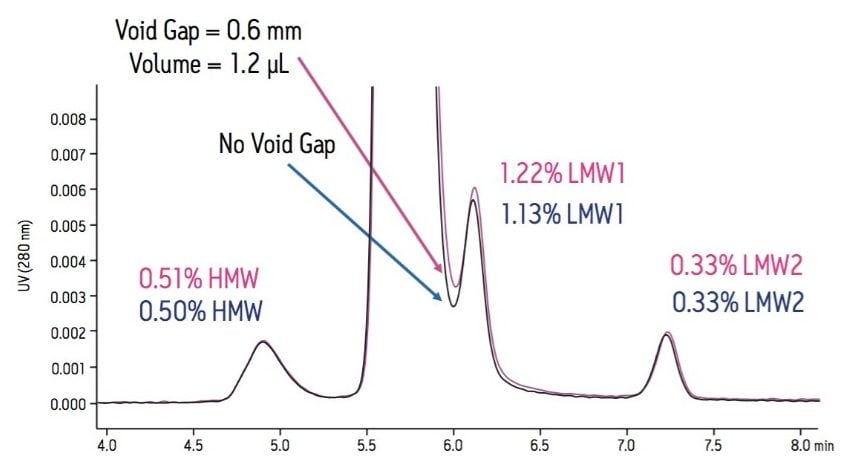
In addition to the capillary tubing used as part of the sample flow path, the selection and correct use of the fittings used to connect the system components and column are of critical importance in SE-UPLC. In this regard, it is important that the cut ends of the tubing be smooth and orthogonal relative to the walls of the tubing. The fittings used prior to the column must be able to withstand the pressures applied during an SE-UPLC analysis (~500 bar) without slipping and creating a void. If permanently swaged fittings are used, it is critical that the tubing and fittings be replaced if the column is replaced to ensure proper seating. An example of how an improper or failed tubing connection can lead to non-reproducible quantitative results is presented in Figure 5. In this example, the intentional generation of a 0.6 mm gap creating a void of approximately 1.2 µL at the connection to the head of the column was made. This alteration resulted in a resolution loss between the monomer peak and the LMW1 from a USP resolution of 1.50 down to 1.46. The loss of resolution between this critical pair resulted in a significant increase (~7%) in the integrated relative area of the LMW1 peak from 1.13% to 1.21%. However, the relative areas of the HMW and LMW2 peaks were not significantly different. This is due to how well the HMW and LMW2 peaks are resolved from the predominant monomer peak and the elution order of the HMW form relative to the monomer, which makes the HMW and LMW2 integration less susceptible to change due to variation in the peak tailing of the monomer. Albeit a predictable result, this example emphasizes the sensitivity that a quantitative SE-UPLC method can have to extra column dispersion.
Once a reliable analytical SE-UPLC method has been developed it may be necessary to transfer that method to a different internal laboratory or external contracted research organization. As shown earlier, a systematic and thorough development process can result in a quantitative method that will not be sensitive to minor variations in mobile phase composition or pH as long as the quality of the columns used is appropriately controlled by the manufacturer.
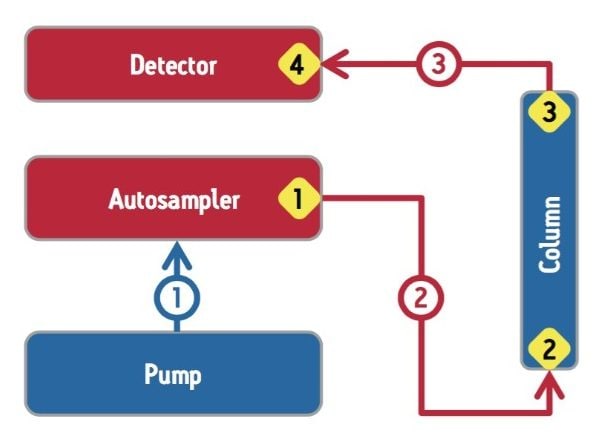
The next major consideration in SE-UPLC method transfer is the extra-column dispersion of the chromatographic systems used in the two labs. In this regard, careful documentation of the system configuration, the critical components and connections (Figure 6), system performance, and the appropriate training of laboratory personnel are critical to the success of the method transfer exercise. In this regard, the use of an appropriate control sample to rigorously evaluate the performance with respect to the extra-column dispersion of the chromatographic system is recommended.
Size-exclusion chromatography continues to be a standard technique for the analysis of monoclonal antibodies and their aggregates. Additionally, as a result of the improved resolutions observed by SE-UPLC, the extent of hinge region fragmentation in the native state of the molecule can also be determined. As in any SEC method, a thorough evaluation needs to be performed to develop an optimum SE-UPLC separation. Conditions that should be systematically evaluated include mobile phase (pH and ionic strength), flow rate, column length, the chromatographic system used and its configuration. The optimization of these parameters for an SEC separation may be evaluated based on critical performance criteria such as resolution, peak tailing, and quantitative reproducibility.
The analytical transfer of an optimized and robust SE-UPLC method may be made successfully. However, the analysts must take care to ensure that the chromatographic systems being used are configured comparably and set up correctly, both initially or when one of the components or columns is replaced, in order to maintain minimal extra-column dispersion.
720004416, July 2012