In this application note, we outlined the use of hexylammonium acetate (HAA) for a variety of oligonucleotide chromatographic applications.
In this application note we describe the preparation of hexylammonium acetate (HAA) and the benefits to its use for a variety of applications compared to TEAA and/or TEA-HFIP for the chromatographic analysis of oligos, including:
Analysis of oligonucleotides via ion-pairing reverse-phase liquid chromatography requires efficient ion-paring systems that yield chromatographic separation primarily based on charge/length.1 The most commonly used ion-pairing agents are triethylammonium acetate (TEAA) and triethylamine hexafluoroisopropanol (TEA-HFIP). TEAA is commonly used due to its low cost and duplex compatability, but suffers from limited resolution of oligonucleotides. TEA-HFIP offers benefits over TEAA, such as increased resolution and predictable retention.
The benefits are the result of increased ion-pairing and a decrease in reversed-phase behavior resulting from the nucleobases, evident in single-stranded oligonucleotide separations. Despite these benefits, the utility of a TEA-HFIP system is somewhat limited. In particular limitations arise for separation of duplex oligonucleotides, because it is denaturing and contributes significantly to melting. The costs associated with the use of TEA-HFIP are significantly higher than those associated with using acetate-buffered mobile phases when considering purchase and disposal.
To overcome some of these issues, we investigated an ion-paring system which uses hexylammonium acetate (HAA). HAA, when used with the Waters ACQUITY UPLC System, provides the same or better resolution of a variety of oligonucleotides, and is non-denaturing for duplexes. The cost associated with the use of HAA is significantly lower than that of HFIP-based mobile phases.
Hexylamine and acetic acid were purchased from Sigma Aldrich. The concentration of HAA in this example is 100 mM single-stranded; however, we have prepared 50 and 10 mM for increased MS-compatibility with little loss in resolution.
1 L of 100 mM HAA is prepared as follows:
It is highly recommended that mobile phase components be measured by mass instead of volume. Significant variation in retention times may result from inaccurate mobile phase preparation.
The mobile phase can be used for several days without any significant loss in oligonucleotide resolution.
Oligonucleotide samples should be dissolved in either 100 mM HAA or 100 mM TEAA, pH 7.0. There is strong affinity of hexylamine for the particle sorbents; therefore, it is highly recommended that a column be dedicated for use with HAA due to its high affinity for the stationary phase.
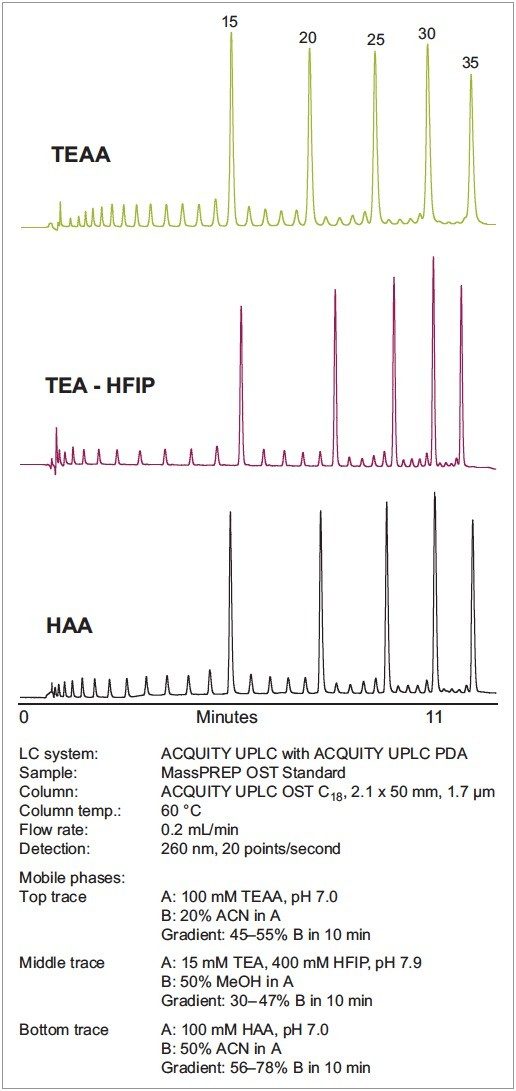
The Waters Oligonucleotide Separation Technology (OST) standard demonstrates the resolving capabilities of HAA compared to traditionally-used mobile phases. The separations shown in Figure 1 were normalized by adjusting each gradient so that the 15-mer and 35-mer peaks eluted at the same time with each mobile phase system. As shown, HAA offers a significant increase in resolution compared to the commonly-used, non-denaturing TEAA mobile phase. The increase in resolution is attributed to the increased ion pairing ability of HAA. Comparing the separation with HAA to the separation with TEA-HFIP clearly indicates that HAA provides similar or better resolution than HFIP-based mobile phase. In fact, our data indicated that the resolution of longer oligonucleotides is better with HAA.
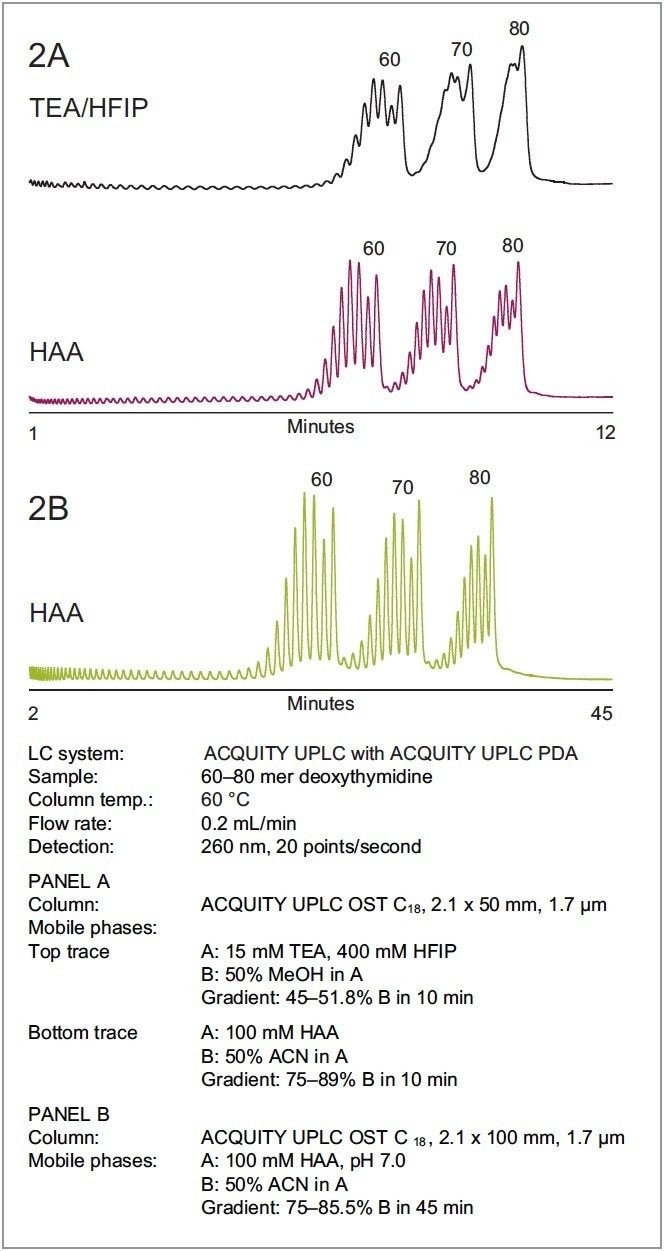
We investigated the increased resolution of longer oligonucleotides with HAA using a sample of 60 to 80 mer deoxythymidines. Figure 2A illustrates the separation of a 60 to 80 mer deoxythymidine ladder performed with TEA-HFIP, top trace, and with HAA, bottom trace. The two separations were normalized by adjusting the gradient of each system so that the retention of the 60 and 80 mer occur at approximately the same time, which allows direct comparison of their resolving power. As shown, there is a significant increase in resolution for long oligonucleotides with HAA compared to TEA-HFIP. Resolution can be increased further by increasing the column length (Figure 2B), but the gradient time must also be scaled proportionally.
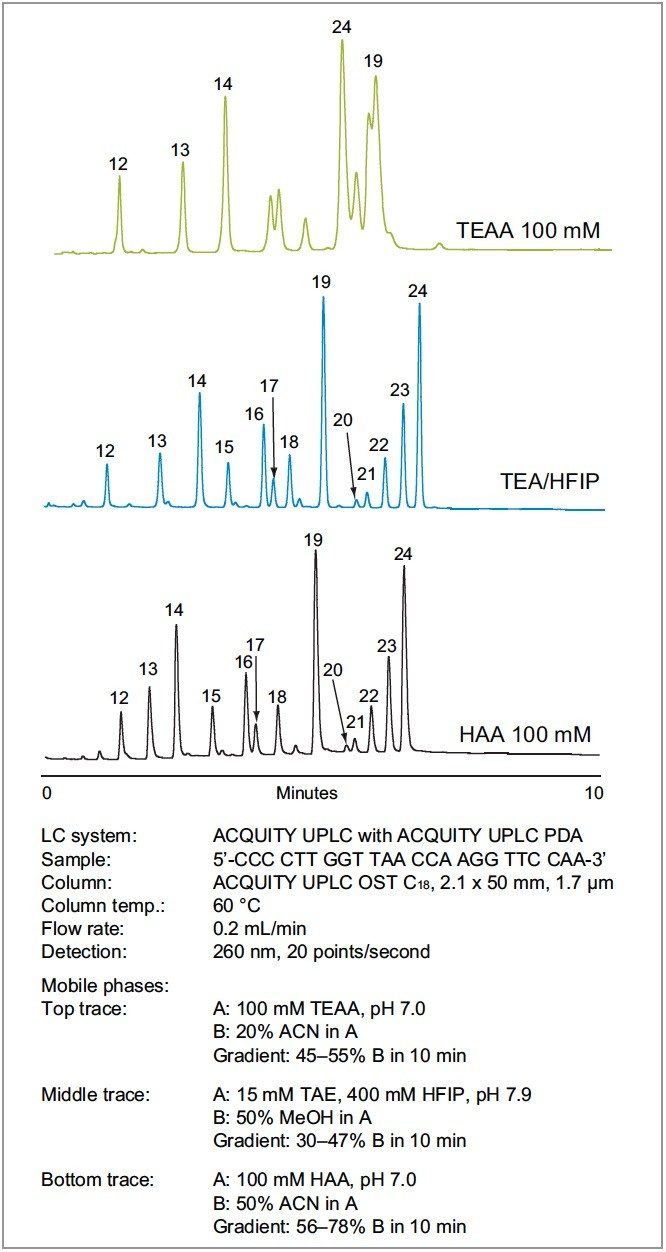
Since most synthetic oligonucleotides used for therapeutic and diagnostic purposes are composed from a variety of bases, we investigated the separation of a more realistic sample of a heteromeric oligonucleotide. For this purpose we used the same chromatographic conditions used in Figure 1 to provide the most fair comparison.
As shown in Figure 3, TEAA does not effectively separate the heteromeric oligonucleotides. In contrast, both HAA and TEA/HFIP give impressive separations. Careful inspection of the data reveals that the separations are virtually identical. The increased chromatographic resolution obtained with HAA and TEA-HFIP compared to TEAA is the result of increased ion-pairing behavior and decreased hydrophobic contribution of the nucleobases. For applications where MS analysis is not required, the use of HAA is a cost-effective alternative that provides the same or better resolution.
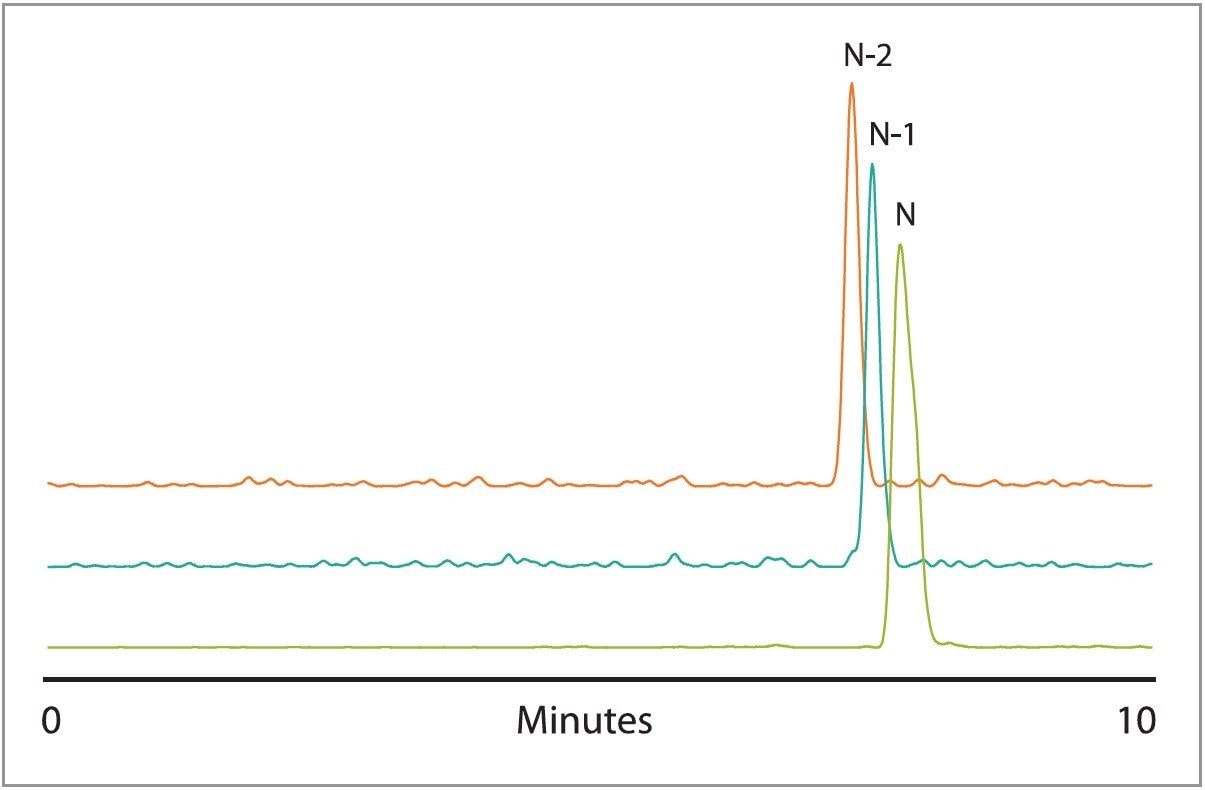
The separation of phosphorothioates can be very challenging. This is largely due to the inherent chirality of the phosphorothioate linkage, which often leads to broad peaks and incomplete resolution of trunca ted sequences due to isomeric distribution. Figures 4 and 5 show that HAA adequately resolves truncated phosphorothioate sequences from the full-length sequence. The ability to resolve N-1 phosphorothioates indicates that HAA partially suppresses diastereomeric resolution. While the MS sensitivity resulting from the use of HAA is lower than that of TEA-HFIP, useful MS data is easily obtained and confirms the separation of the full-length phosphorothioate from truncated sequences as shown in the selected ion chromatograms in Figure 5.
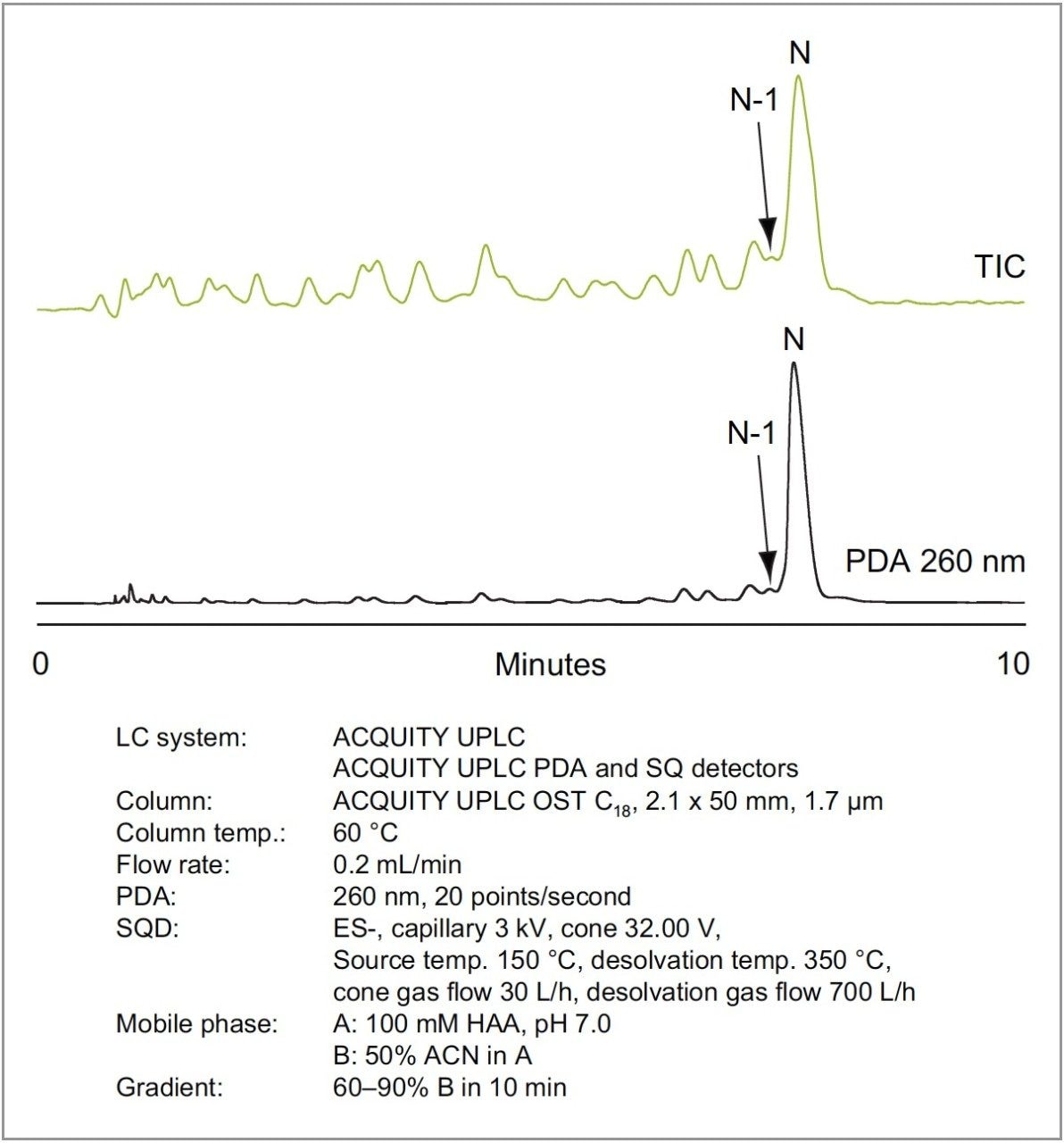
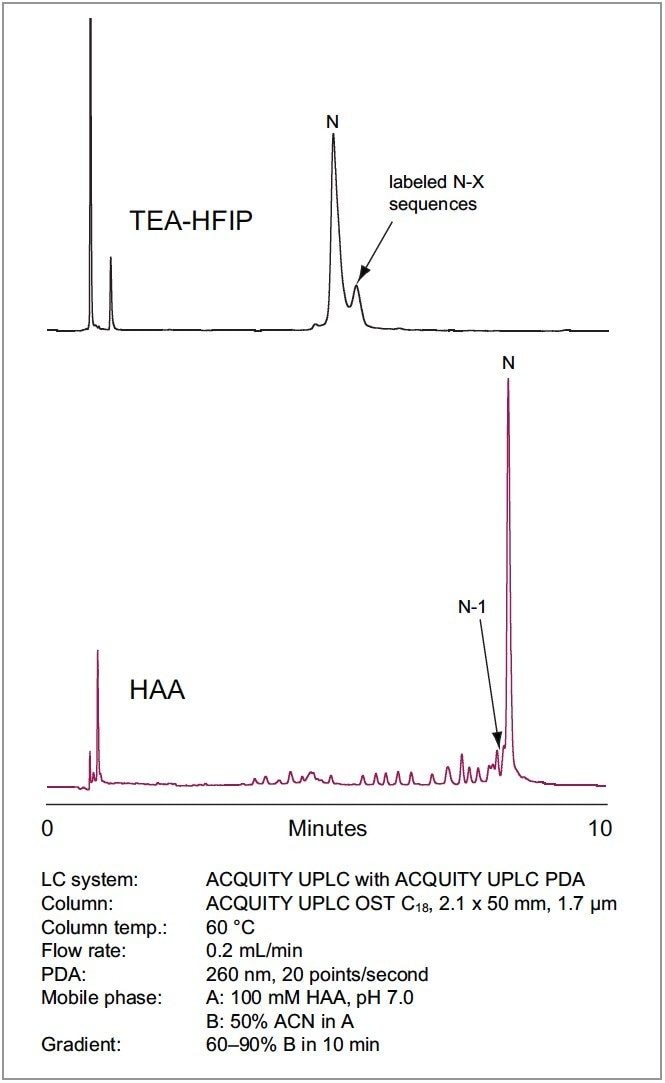
Many oligonucleotides are labeled with fluorescent tags for a variety of reasons. These tags are generally large aromatic and poly-aromatic groups that impart significant hydrophobicity to the oligonucleotide. For this reason, inefficient ion pairing often leads to poor resolution of labeled species as hydrophobic forces overcome the ion-pair mechanism. Figure 6 illustrates the analysis of a hydrophobically-labeled oligonucleotide with TEA-HFIP (top trace) and HAA (bottom trace). TEA-HFIP does not yield the desired elution pattern. Instead, the target peak is followed by truncated labeled species. The reversal in elution order results from increased contribution of the hydrophobic tag to chromatographic behavior as the hydrophilic oligonucleotide size decreases. The use of a more efficient ion-paring agent, such as HAA, results in regular retention of all labeled species in their expected order.
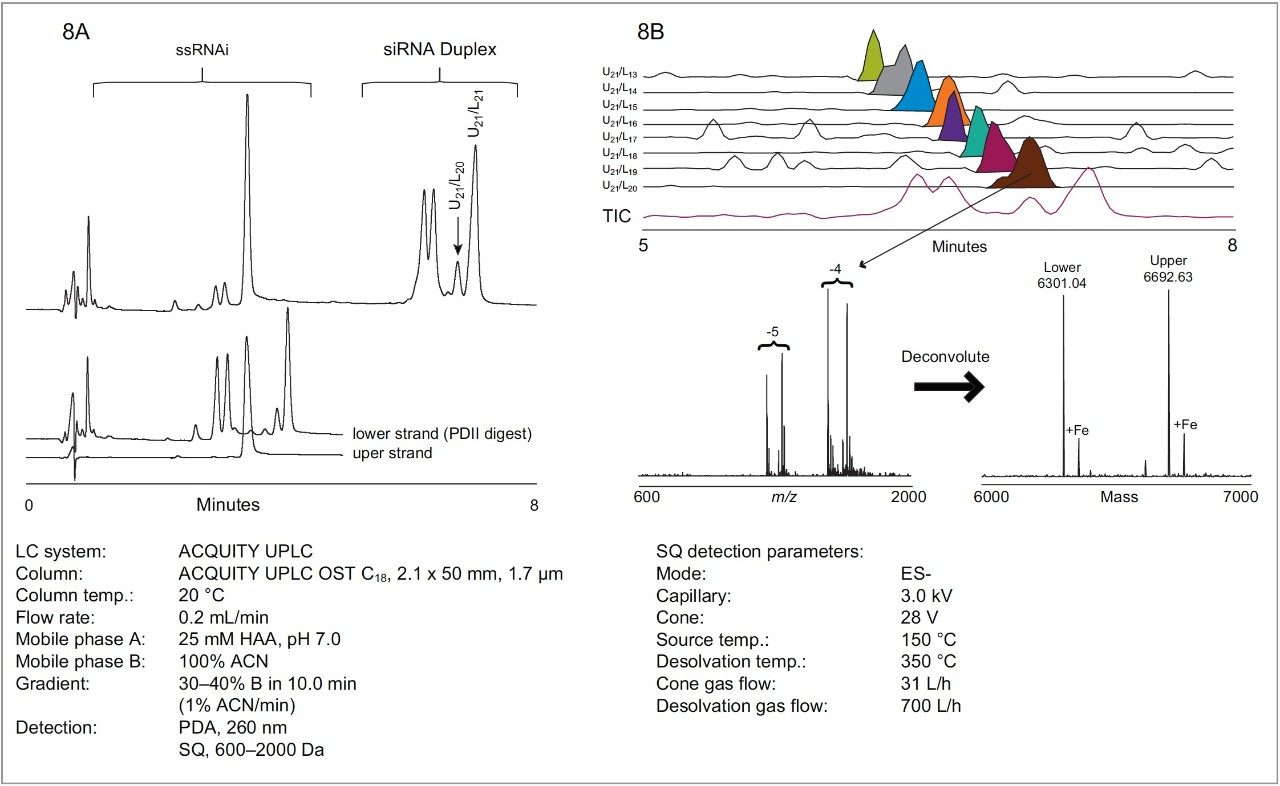
As mentioned previously, HAA is non-denaturing and is fully compatible with oligonucleotide duplexes. We investigated the ability of HAA to resolve mismatched from full-length RNA duplexes. To demonstrate the ability of HAA to resolve RNA duplexes, we utilized a purified upper RNAi strand and a partially-digested lower strand. Upon annealing, a ladder of siRNA duplexes is formed with partially 5' truncated lower RNAi strands (Figure 7).
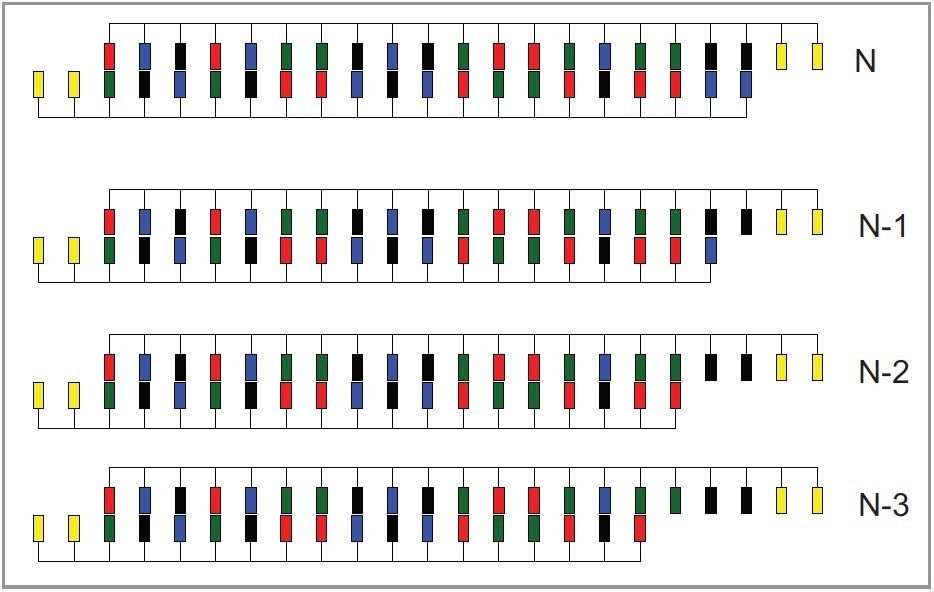
As shown in Figure 8A, HAA successfully resolves truncated siRNA duplexes from full-length duplex and single-stranded RNAi species. Using hexylammonium acetate we were also able to identify each eluting duplex peak by the corresponding mass of complementary RNAi strands with MS. In this way we were able to confirm the elution order of the impurities. Extracted selected ion chromatograms shown in Figure 8B indicate resolution of truncated duplexes.
In this application note, we outlined the use of HAA for a variety of oligonucleotide chromatographic applications. HAA and TEA-HFIP exhibit similar resolution of moderate length, unmodified homo and heteromolecular oligonucleotides. For separation of longer oligonucleotides, ca. >35-mer, HAA exhibits better resolution which can be further improved with longer columns and correspondingly longer gradient times. HAA is able to adequately resolve phosphorothioates, a particularly difficult separation. The separation of labeled oligonucleotides shows a clear advantage of HAA over TEA-HFIP with HAA giving predictable retention of labeled species and minimal contribution of the hydrophobic label. Finally, the non-denaturing character of HAA allows for its use with oligonucleotide duplexes and offers impressive resolution of mismatches. While HAA does not offer the same MS compatibility as TEA-HFIP, its utility is evident. Taken together this data indicates that the use of HAA with the ACQUITY UPLC System provides exceptional separation of a variety of oligonucleotides at a significantly lower cost than TEA-HFIP.
720003361, November 2016