The introduction of BEH SEC, 3.5 μm, HPLC-compatible columns with a pore-size of 125Å provides improved resolution SE-HPLC separations in comparison to traditional 5 μm silica-based particles. This characteristic, in combination with the chemical stability of BEH Technology, provides outstanding column lifetimes. As part of the Waters Protein Separation Family of columns, these columns are manufactured to rigorous tolerances and quality tested with relevant analytes. These HPLC separations are also directly scalable to SE-UPLC separations using 1.7 μm diameter BEH technology particles and narrower column internal diameters (4.6 mm I.D.), which have even greater resolution and samplethroughput when coupled with UPLC-capable chromatographic systems.
Waters currently provides the only sub-2-µm particle size size-exclusion chromatography (SEC) column with a pore-size (125Å) suitable for the analysis of small proteins and peptides.1 This UPLC Technology size-exclusion (SE-UPLC) column consists of 1.7 µm diameter ethylene bridged hybrid (BEH) particles, which are mechanically strong and more chemically stable than pure silica-based particles. However, the small particle-size and narrow 4.6 mm internal diameter of SE-UPLC columns are not optimal for use with an HPLC system due to the significantly higher extra-column dispersion and lower pressure limits of HPLC systems relative to UPLC systems. As a result, Waters has recently developed a 125Å pore-size, 3.5 µm particle-size BEH SEC column specifically for use on classic HPLC instrumentation. This provides laboratories with HPLC instrumentation a means to take advantage of the benefits provided by BEH particle technology. This application note will cover the performance characteristics of this column, designed for the separation of small proteins and peptides, with respect to UPLC method transfer, column-to-column reproducibility, and column stability. Additionally, the performance advantages that this 3.5 µm packing material offers over larger (5 µm), standard SE-HPLC particles for both non-denaturing and denaturing separations will be highlighted.
(unless noted otherwise)
|
LC systems: |
Alliance HPLC, ACQUITY UPLC H-Class Bio System, or ACQUITY UPLC |
|
Detection: |
Alliance HPLC TUV Detector, ACQUITY UPLC TUV Detector with 5 mm titanium flow cell |
|
Wavelength: |
280 or 214 nm |
|
Columns: |
XBridge Protein BEH SEC, 125Å, 3.5 μm, 7.8 x 300 mm (p/n: 176003596) and ACQUITY UPLC Protein BEH SEC, 125Å, 1.7 μm, 4.6 mm x 300 mm (p/n: 186006506) |
|
Comparator columns: |
Silica-diol SEC, 125Å, 5 μm, 7.8 x 300 mm |
|
Column temp.: |
Ambient |
|
Sample temp.: |
10 °C |
|
Injection vol.: |
10 μL (unless otherwise noted) |
|
Flow rate: |
0.84 mL/min (unless noted otherwise) |
|
Mobile phases: |
25 mM sodium phosphate, 150 mm sodium chloride, pH 7.2 (prepared using Auto•Blend Plus Technology) |
|
Mobile phase A: |
100 mM NaH2PO4, 14.5% |
|
Mobile phase B: |
100 mM Na2HPO4, 15.0% |
|
Mobile phase C: |
1.0 M NaCl, 65% |
|
Mobile phase D: |
Water or 30% (v/v) acetonitrile, 0.1% (v/v) TFA |
|
Gradient: |
Isocratic |
|
Standard: |
BEH125 SEC Protein Standard Mix (p/n: 186006842) |
|
Sample Vials: |
Deactivated Clear Glass 12 x 32mm Screw Neck Total Recovery Vial, with Cap and preslit PTFE/Silicone Septa, 1 mL (p/n: 186000385DV) |
|
Chromatography software: |
Empower Pro (v2 and v3) |
All samples were diluted in mobile phase unless otherwise noted. Proteins and peptides were purchased as individual standards or as mixtures (Waters and Sigma-Aldrich). Sample concentrations were 1.0 mg/mL (nominal) unless noted otherwise.
The significant sample throughput and performance benefits provided by BEH particle technology when used in the manufacturing of size-exclusion UPLC (SE-UPLC) columns for the analysis of peptides and proteins have been previously described.2,3 However, these advantages cannot be fully realized when using HPLC instrumentation due to the peak dispersion introduced by these systems. In order to take advantage of the chemical and structural capabilities of BEH particle technology for the SEC separation of small proteins and peptides on HPLC instrumentation, 7.8 mm I.D. columns packed with 3.5 µm BEH particles with a pore size of 125Å has been recently introduced. This column provides an optimal molecular weight range of SE-HPLC separations to include protein and peptide with radii of hydration (Rh), that translates to a molecular weight range from <1 KDa to approximately 80 KDa. As part of this evaluation, the separation efficiency advantages of this packing material with respect to larger particle-size (5 µm) HPLC-based packing materials, and the critical performance characteristics of column-to-column reproducibility and column stability (i.e. lifetime) will be demonstrated.
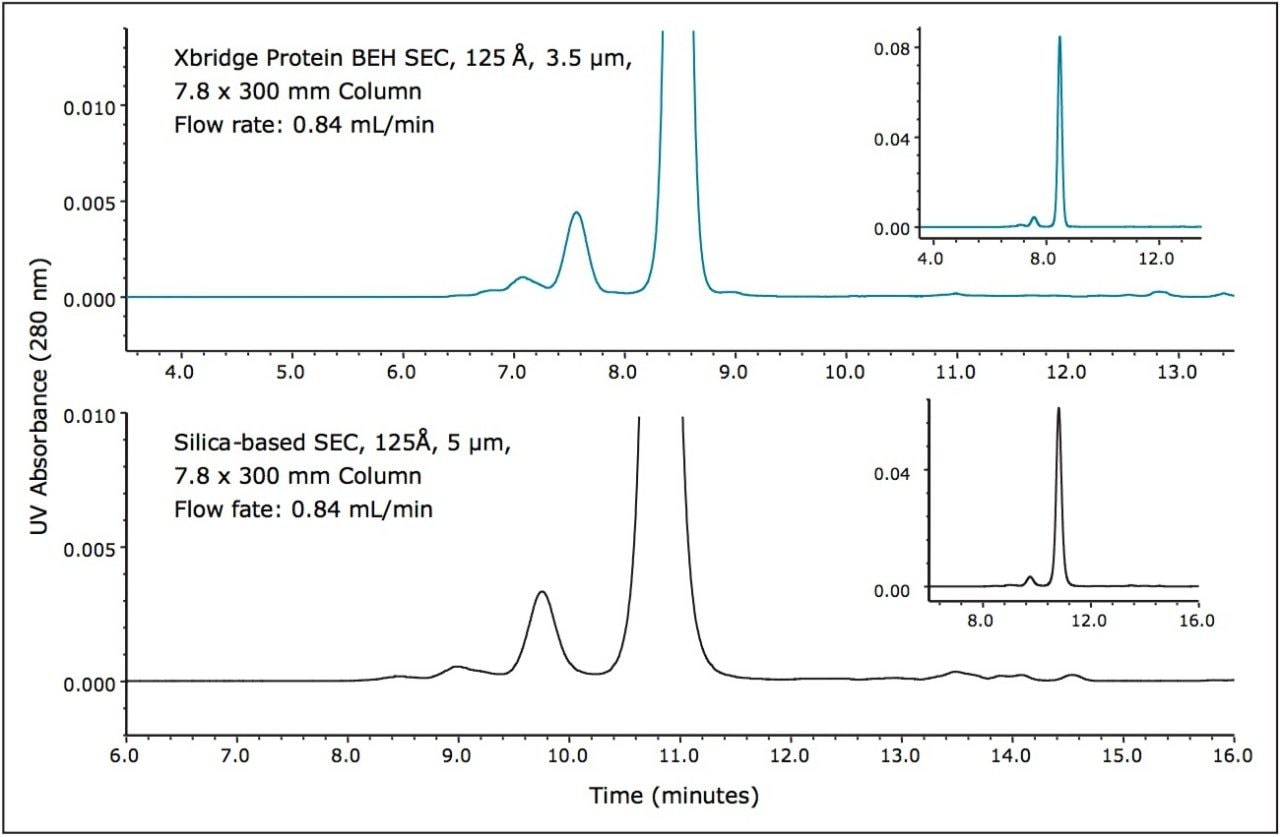
To demonstrate their performance, a small protein (myoglobin, MW 17 KDa) and a series of peptides were separated on a silica-based SEC, 125Å, 5 µm, 7.8 x 300 mm column and on a BEH SEC, 125Å, 3.5 µm, 7.8 x 300 mm column using the same Alliance HPLC System and either aqueous or organic mobile phase conditions (Figures 1 and 2). Equivalent flow rates and injection volumes were used for both comparisons. Improved sensitivity and narrower peak widths were observed on the 3.5 µm packing material in comparison to the 5 µm particle size materials. The BEH SEC column has improved mechanical properties that allow operation at higher flow rates and pressures than can be tolerated by traditional SE-HPLC columns.
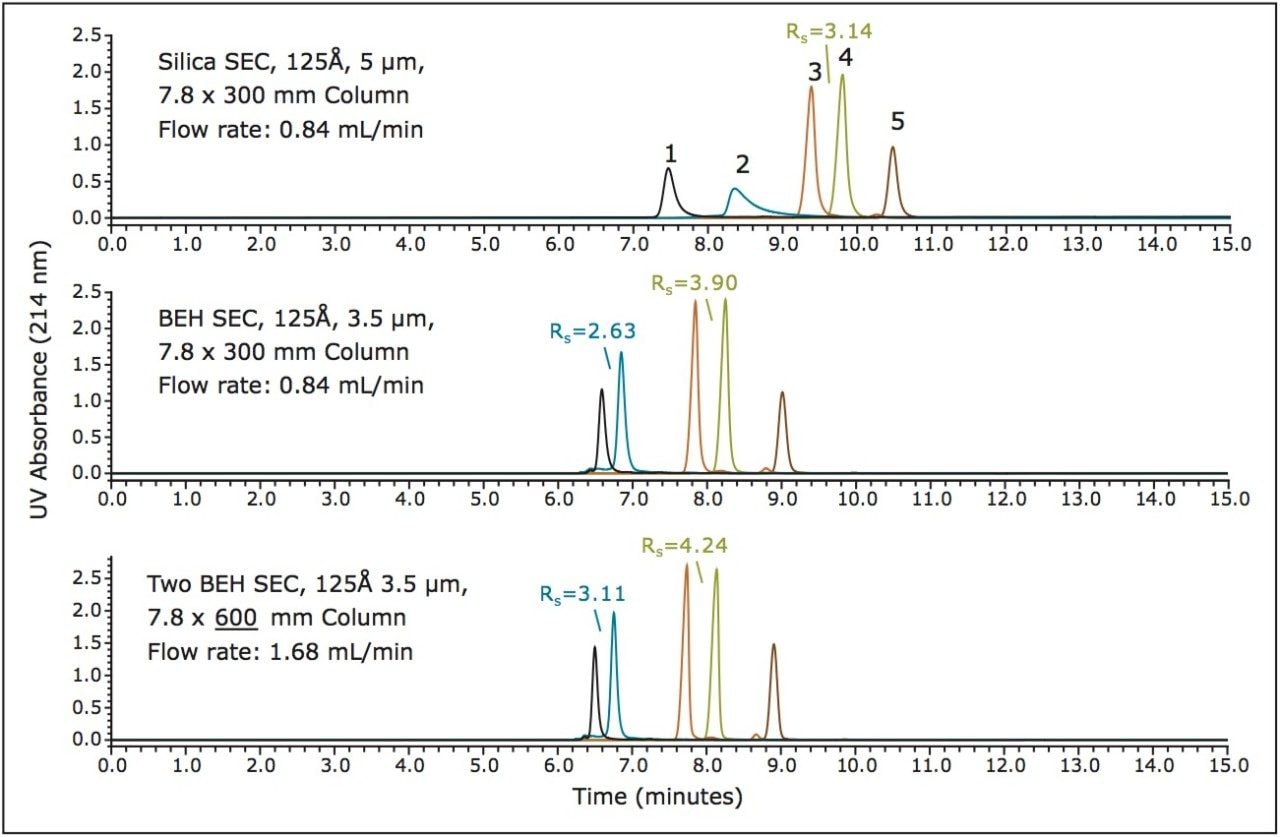
An example of how these capabilities can be used to advantage is shown in Figure 2 (bottom frame). For this analysis two BEH SEC, 125Å, 300 mm columns were operated in series (3.5 µm, 7.8 x 600 mm total length) and by increasing the flow rate two-fold a significant increase in resolution can be observed while maintaining equivalent analysis times. Another advantage that can be observed by employing BEH particles for the SEC separation of peptides is a reduced level of secondary interactions for certain peptides (Figure 2) such as ubiquitin and aprotinin in comparison to the secondary interactions observed for the silica-based SEC column. These secondary interactions are likely the result of silanol activity given the charge characteristics of these two peptides. Aprotinin is a very basic protein (pI = 10.5) and ubiquitin presents a highly basic surface charge with multiple lysines that are involved in the protein-ubiquitin interactions referred to as ubiquination.
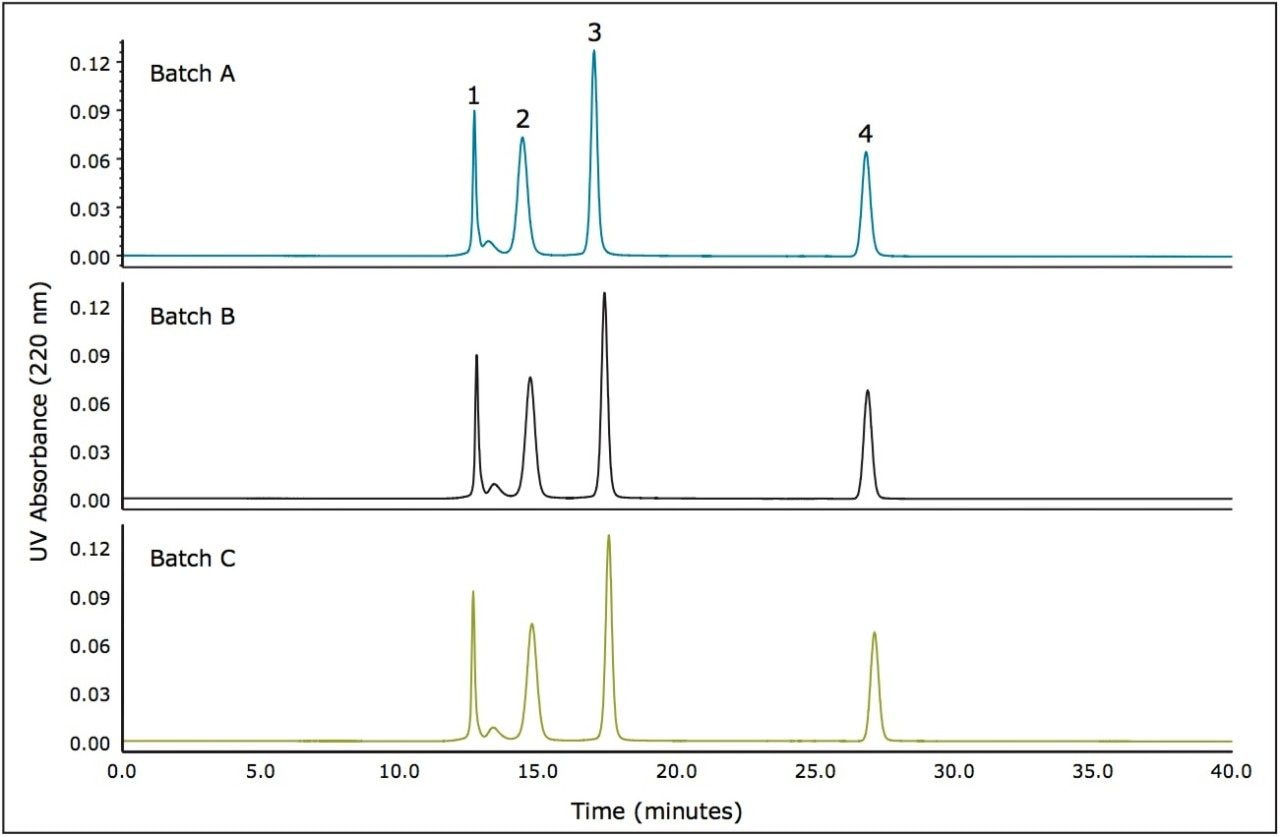
Major concerns for method development are batch-to-batch and column-to-column reproducibility, as well as packed column stability. Shown in Figure 3 is an overlay of the chromatograms for a series of molecular weight standards separated on three 125Å, 3.5 µm, 7.8 x 300 mm columns. These chromatograms demonstrate the reproducibility of these SEC columns packed from 3 different production lots of packing material. At a flow rate of 0.42 mL/minute, the retention time standard deviations for the 125Å pore size columns ranged from a minimum of 0.06 minutes to 0.27 minutes with an average standard deviation of 0.17 minutes for all labeled components. The average retention time reproducibility relative to the retention time of uracil (total permeation volume) was 0.99% RSD.
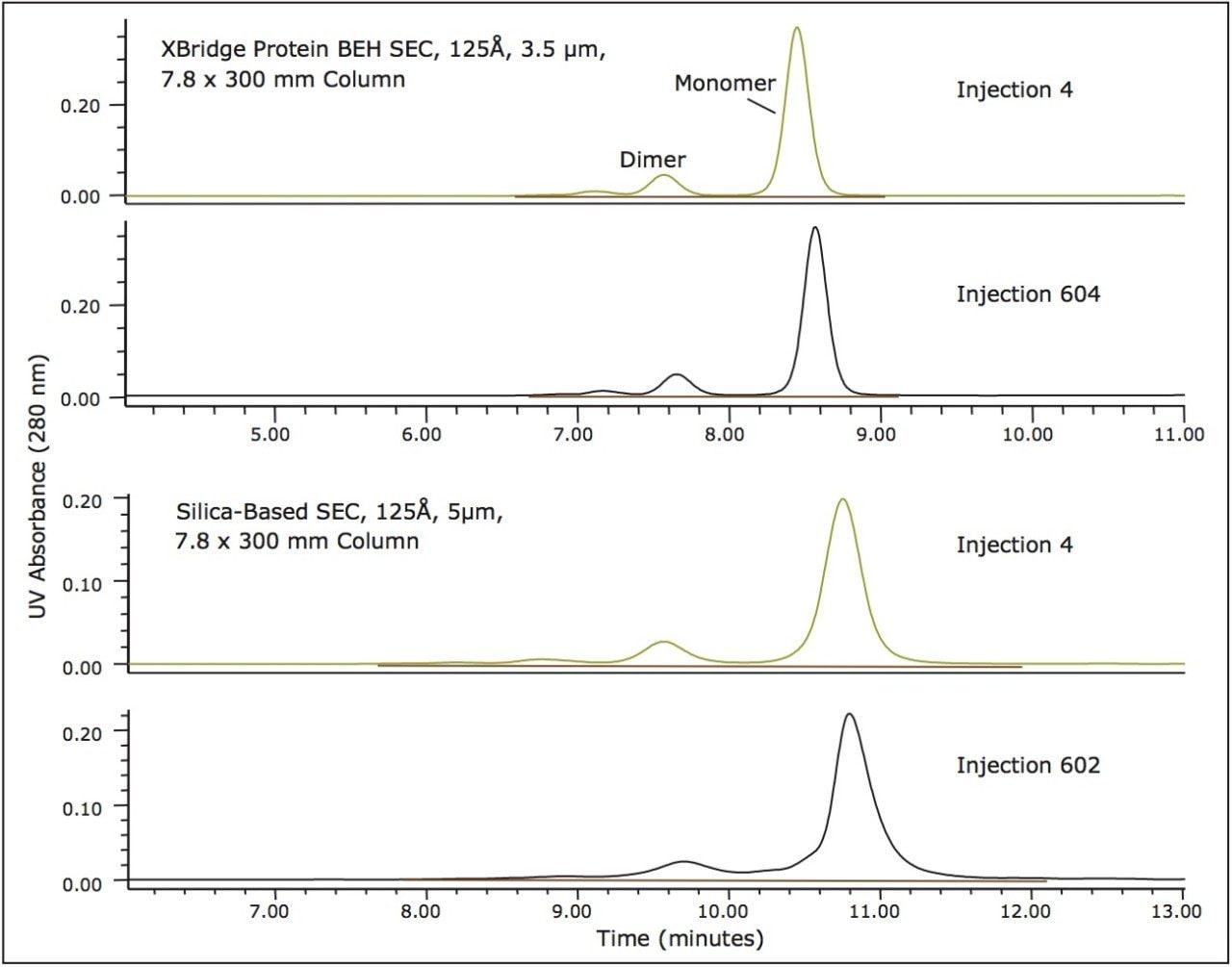
The stability of the BEH SEC, 125Å, 3.5 µm, 7.8 x 300 mm column can be demonstrated by evaluating the results for a protein standard over the course of over 600 total injections. Given that the stability of silica-based SEC columns can be deleteriously altered by mildly basic pH levels, the pH of the mobile phase was set to 7.2, equivalent to that of phosphate buffered saline (PBS) buffer. Shown in Figure 4 is a comparison of the profiles obtained for the myoglobin standard from the start to the finish of the study for both the BEH SEC, 125Å, 3.5 µm column and a traditional pure silica-based, 125Å, 3.5 µm column. The resolution between the critical myoglobin monomer and dimer peaks were determined for each column. The BEH 125Å, 3.5 µm column demonstrated remarkable stability with no significant depreciation of resolution. These data demonstrate that XBridge Protein BEH SEC, 3.5 µm Columns can provide the reproducibility and stability needed to develop reliable assays and run them routinely in a quality control environment.
Successful method transfers between UPLC and HPLC platforms for both 200Å and 450Å pore-size SEC columns have been described previously.3 The transferability of an SEC method between UPLC and HPLC platforms will be primarily impacted by the comparability of the pore-size and surface chemistry of the particles. For example, in comparing the peptide separations on the BEH and silica-based SEC columns (Figure 2) it is clear that under equivalent conditions that the secondary interactions observed for the peptides ubiquitin and aprotinin are not equivalent between the two particle surfaces. An exercise was undertaken to demonstrate the method transfer between an XBridge Protein BEH SEC, 125Å, 3.5 µm Column and an ACQUITY UPLC Protein BEH SEC, 125Å, 1.7 µm Column. Based on general chromatographic scaling principles it was determined that the column length for the HPLC column would need to be twice that of the UPLC column in order to maintain a comparable column length to particle diameter ratio (L/dp) and the reduced linear velocity would need to be half that of the UPLC Column. The result of this method transfer is shown in Figure 5. The observed profiles are comparable between the two separations when the time-axis is normalized, however, it should be noted that the analysis time for the 3.5 µm HPLC separation using a 7.8 mm I.D. column, takes 4 times longer than the separation on the 1.7 µm UPLC separation using a 4.6 mm I.D. column and uses nearly 6 times more mobile phase. By contrast, to appropriately scale a 1.7 µm, 4.6 mm SEC separation to a 5 µm, 7.8 mm HPLC column would require the use of three times the column length and the run-time would be approximately nine times longer.

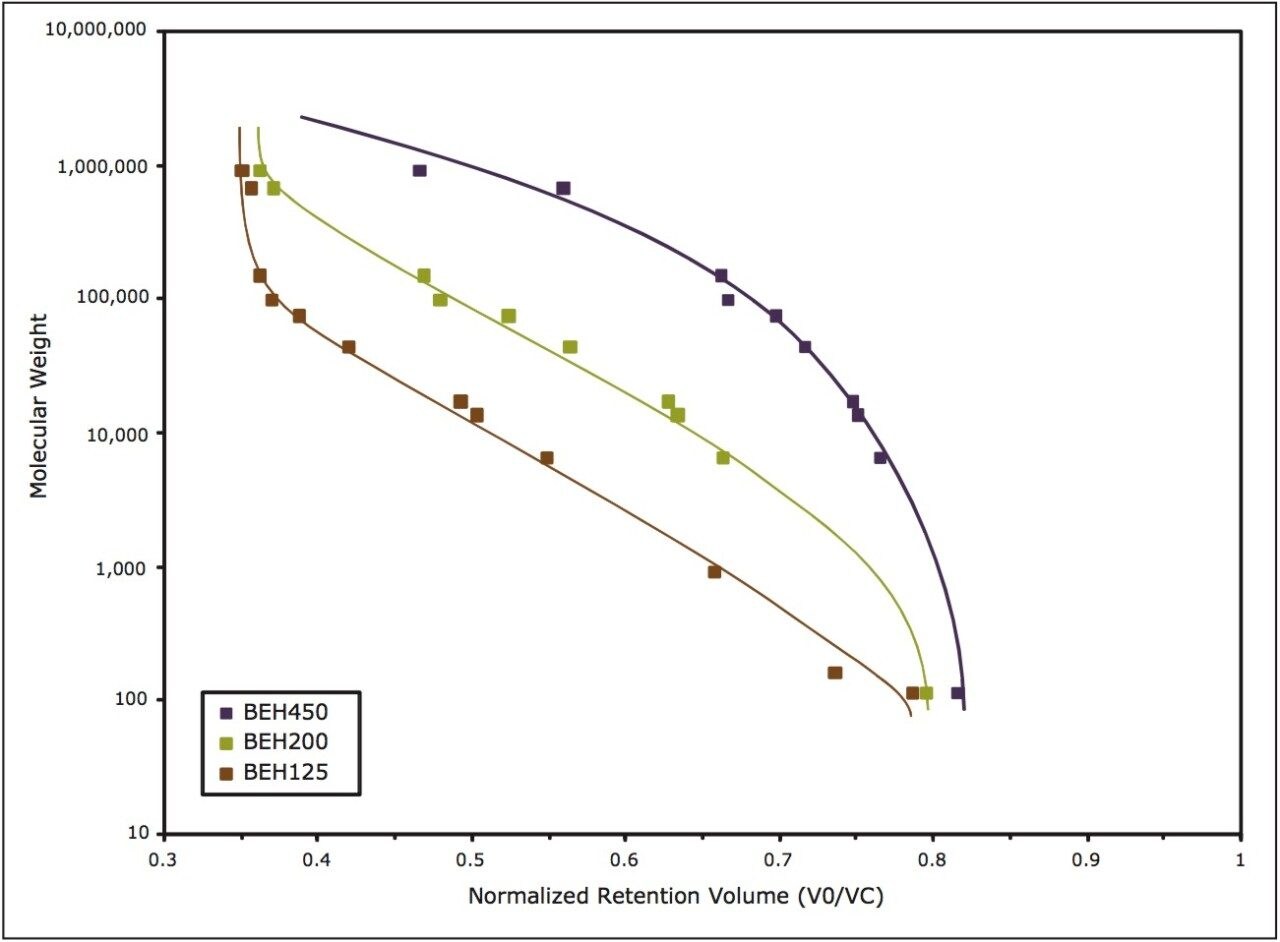
Comparisons were made between the 125Å, 200Å, and 450Å pore-size particles for the separation of proteins and peptides. The protein molecular weight calibration curves are shown in Figure 6. The linear molecular weight range for the 125Å pore-size column is estimated to be from approximately <1 KDa to 80 KDa, the 200Å pore-size ranges from 10 KDa to 450 KDa, and the 450Å pore-size column is estimated to be from approximately 50 KDa to over 1.3 MDa.
The introduction of BEH SEC, 3.5 µm, HPLC-compatible columns with a pore-size of 125Å provides improved resolution SE-HPLC separations in comparison to traditional 5 µm silica-based particles. This characteristic, in combination with the chemical stability of BEH Technology, provides outstanding column lifetimes. As part of the Waters Protein Separation Family of columns, these columns are manufactured to rigorous tolerances and quality tested with relevant analytes. These HPLC separations are also directly scalable to SE-UPLC separations using 1.7 µm diameter BEH technology particles and narrower column internal diameters (4.6 mm I.D.), which have even greater resolution and sample-throughput when coupled with UPLC-capable chromatographic systems.
The Waters XBridge Protein BEH SEC, 125Å, 3.5 µm Column provides:
720005369, June 2016