
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the improved performance of Alliance HPLC System with appropriate syringe, sample vials, reservoir caps, and recommended method settings for meeting the relative standard deviation system suitability requirements of the selected eight molecules.
HPLC has been established as a gold standard in the pharmaceutical industry. The main purposes for using HPLC are for identifying, quantifying, and purifying the individual components of the mixture. HPLC plays an important and critical role in the field of pharmaceutical industries and analysis, since it is used to test the products and to detect the raw ingredient used to make them i.e., qualitative and quantitative analysis. Moreover, the importance of HPLC uses in these fields falls under the stringent regulations established by the U.S. Food and Drug Administration (FDA). This obligates all pharmaceutical companies to detect the quality of their products by using the HPLC system before allowing them to sell it in the global market. The pharmaceutical products are evolving day by day and at the same time regulatory demands are increasing too; at times this poses challenges for HPLC and meeting the criteria of system suitability. It is important to understand the regulation and find a solution to address such requirements.
In this experiment, eight challenging pharmacopoeial and non-pharmacopoeial assay methods as listed in Table 1 were selected based on their injection volume, either 5 or 10 µL, which is lower than typical HPLC injection volume in combination with 100% organic solvent as diluent. Lower injection volume and 100% organic solvent as diluent poses a unique challenge of area precision in HPLC for extended sequence due to longer run time and/or several batches needed to be run back-to-back. All three combinations explained above can make the relative standard deviation for standard and bracketing standard injections area values run out of acceptance window leading to reanalysis.
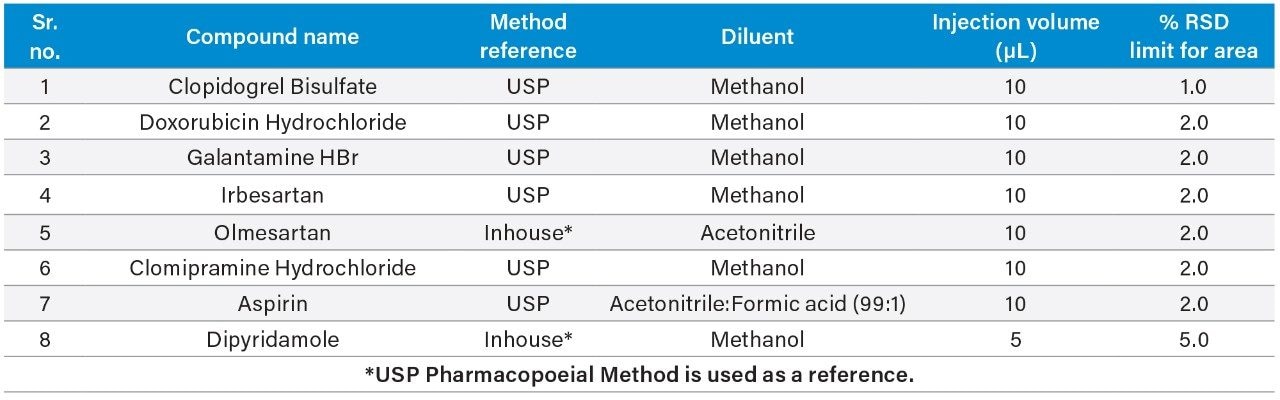
In this paper, we have demonstrated the improved performance of Alliance HPLC System with appropriate syringe, sample vials, reservoir caps, and recommended method settings for meeting the relative standard deviation system suitability requirements of the selected eight molecules.
For increased injection volume accuracy, Alliance HPLC System was configured with 100 µL syringe. As all the selected methods contain 100% organic solvent as diluent, sample vials with specific self sealing septa were used to avoid evaporation and pressurization of the vials, vial caps were screwed only up to resistance. The syringe draw rate in instrument method was selected as fast and to avoid the evaporation of mobile phase solvents, specific reservoir caps were also used.
Eight pharmaceutical assay methods were analyzed in the Alliance HPLC System with described conditions, and data was acquired as a typical quality control assay sequence. Six replicates of standard were made followed by six test sample injections, and bracketing standard were injected post test sample. Five to six such sample test sets were injected in each sequence and data was recorded as per individual method conditions.
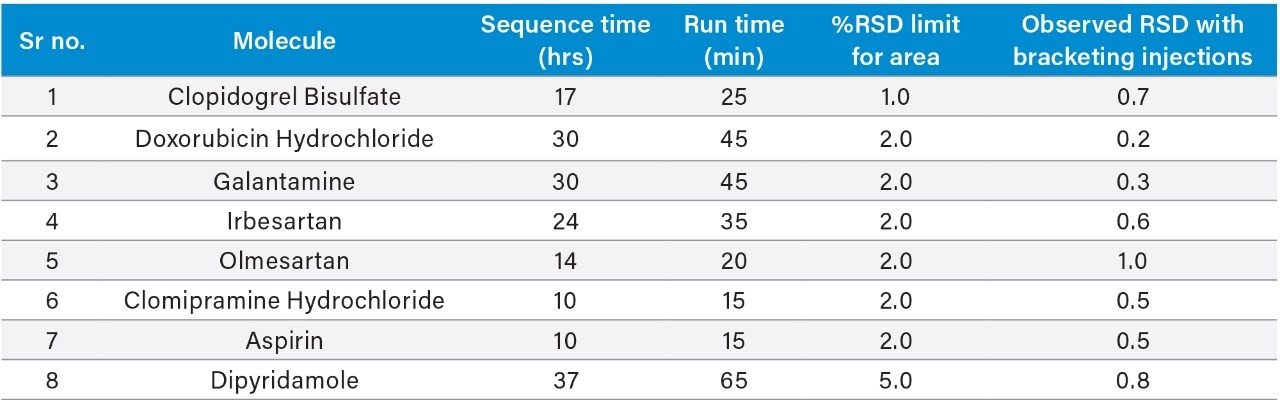
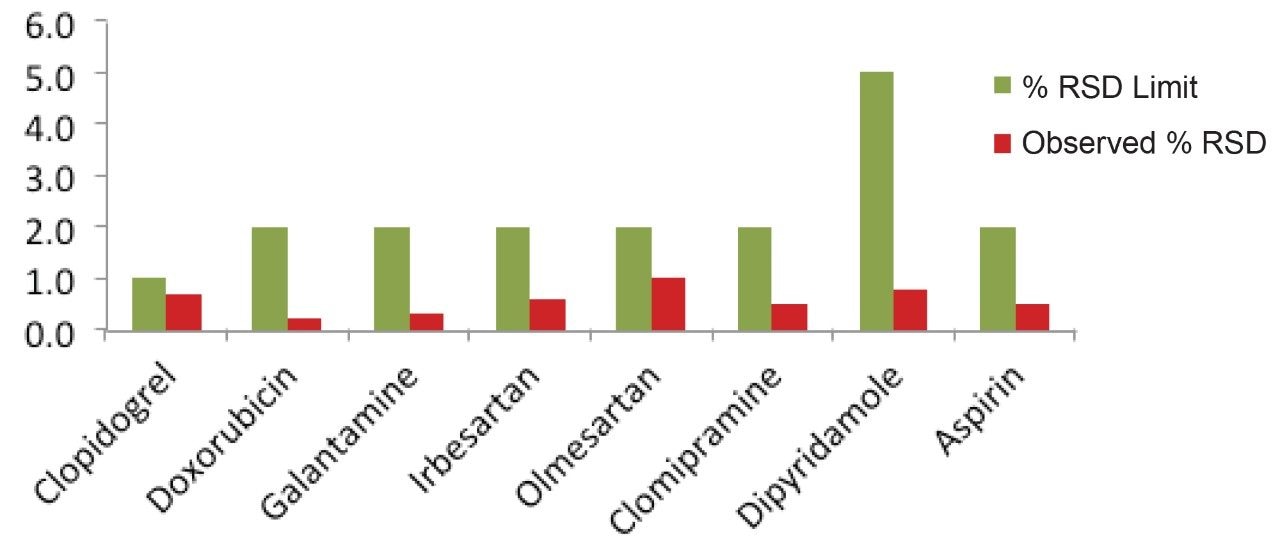
The acquired data of each method was processed with a single processing method and results were generated. The results obtained for each method were compiled in Table 2. The relative standard deviation values of area for all of the eight methods were found to be well within the specified limit of system suitability test for respective method.
The Alliance HPLC System with 100 µL syringe and method specific precaution can be used for routine quality control analysis of the selected eight methods. Improved performance of the Alliance HPLC System demonstrated the system capability to address the system suitability criteria of area precision for eight challenging pharmacopoeial and non-pharmacopoeial assay methods.
720006125, November 2017